Editorial on “Adjuvant treatment for high-risk clear cell renal cancer: updated results of a high-risk subset of the ASSURE randomized trial”
We read with great interest the paper entitled “Adjuvant Treatment for High-Risk Clear Cell Renal Cancer Updated Results of a High-Risk Subset of the ASSURE Randomized Trial” by Haas et al. (1). Last year, the same group published the results of a randomized phase III trial ASSURE comparing 1-year treatment with sorafenib (400 mg twice daily), sunitinib (50 mg/day for 4 weeks of every 6 weeks), or placebo as adjuvant therapies for patients with completely resected renal cell carcinoma (RCC), without reporting significant improvements of the disease-free survival (DFS) in the study arms (2). More recently, Ravaud and his group firstly showed the results of a 750-patient randomized study, S-TRAC (3), (sunitinib 50 mg daily with 4/2 schedule vs. placebo in clear cell RCC predominant pT3-4 or node-positive disease). The authors showed an advantage in terms of DFS of 1.2 years (6.8 vs. 5.6 years) in this population of patients with high-risk of recurrence treated with sunitinib as adjuvant therapy compared to placebo, without mature results on overall survival (OS) at time of data cut-off (3).
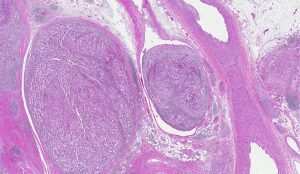
Based on these new evidences, Haas et al. performed an updated analysis (1) of data from the Assure trial (2) focusing on the high-risk population. This subgroup was composed by patients with pT3 and higher stage (with tumor growing into a major vein, such as the renal vein or the vena cava, or into tissue around the kidney, but not invading the adrenal gland or overcoming beyond Gerota’s fascia, Figure 1) or node-positivity. Differently from S-TRAC study (3), they did not find a significant benefit in terms of DFS (5-year rates were 47.7%, 49.9%, and 50.0% for sunitinib, sorafenib and placebo, respectively) or OS (5-year rates: 75.2%, 80.2% and 76.5%) by treating patients with anti-vascular endothelial growth factor receptor (VEGFR) tyrosine kinase inhibitors (TKIs) compared to placebo (1).
So how can we explain the different results obtained by these two studies? Should we treat or not patients with high-risk of recurrent RCC after nephrectomy with sunitinib or do we need more data to better identify the subpopulation of patients who will certainly benefit from this approach? Firstly we can start analysing this questions underling the different population enrolled in these two studies. Indeed, the ASSURE trial included also patients with non-clear cell histology, who represented more than 20% of the whole study accrual (1) and were excluded from the S-TRAC trial (3). Moreover, risk assessment was calculated following AJCC indications (1), while in the S-TRAC study it was used the UISS system (3). In addition, only 67.7% of patients started at full sunitinib dose in the study by Haas et al. (1) and it was followed to reduce the dose till a minimum of 25 mg, while Ravaud et al. (3) included only patients treated at the beginning with 50 mg of sunitinib, with a maximum reduction allowed till 37.5 mg.
The study by Haas et al. also investigated the impact of receiving higher or reduced doses of sunitinib or sorafenib in terms of patients’ outcome. They reported that starting dose as well as dose reductions were associated with a DFS that didn’t differ from that registered by patients treated with higher doses. These data are consistent with those published by Iacovelli et al. (4), who revealed that toxicity-related dose reductions in 591 patients treated with first-line sunitinib or pazopanib were correlated with longer OS and with a better outcome with second-line treatments (4).
Another very interesting topic is represented by the responsiveness to target agents or immunotherapies of patients with recurrent disease following adjuvant therapy. What we still don’t know is the complex series of changes caused in the tumor microenvironment by adjuvant therapy, which is aimed to prolong the time from nephrectomy to tumor recurrence. In this view, it is important to consider the results published by our group in 2014 focused on the biological features of patients with metastatic RCC relapsed >5 years from nephrectomy (5-7). We showed that this group of patients with a long DFS presented a different pattern of metastatic spread, involving unusual site of metastases, such as stomach and glands (5,6), and were particularly responsive to first-line sorafenib, sunitinib or pazopanib without significant differences (5,6). In late-relapsing patients, inflammation resulted highly prognostic, with patients with higher neutrophil to lymphocyte ratio (NLR) associated with shorter PFS and OS compared to patients with lower NLR (7). Taken together, these data underline the particular biological features that characterize patients with prolonged DFS, thus suggesting that patients with increased DFS due to adjuvant therapy should be carefully studied in order to optimize the diagnosis and to select to most potentially effective strategies at tumor recurrence. At this regard, a phase II study (NCT01649180, NEXT, PrE0801) was planned to assess the efficacy of anti-VEGFR TKI axitinib at recurrence after adjuvant therapy in RCC.
Based on the results obtained by Nivolumab in patients with metastatic RCC (8,9), the use of immune checkpoint inhibitors in the adjuvant setting should be carefully evaluated. A reason to be potentially optimistic is the ability of anti-PD-1 and anti-PD-L1 inhibitors to shape memory phenotype CD8 T cell subsets (10). At present, four different phase III studies are investigating the efficacy and tolerability of Pembrolizumab (NCT03142334, KEYNOTE-564), Atezolizumab (NCT03024996, IMmotion010), Durvalumab alone or with anti-CTLA-4 tremelimumab (NCT03288532) and the combination of Nivolumab and Ipilimumab (NCT03138512, CheckMate 914) as adjuvant therapy in patients with RCC. These studies are actively enrolling at this time and the results are awaited in the next 5 years. An evolution of PD-1/PD-L1 approach is under evaluation in the URroRCC study (NCT02429440), a phase I/II trial that will test the efficacy and safety of intradermal application of adjuvant peptide vaccine (developed by using tumor associated peptides) in combination with either granulocyte macrophage colony stimulating factor (GM-CSF) or Montanide ISA-51 in patients with clear cell and not-clear cell RCC histology.
In conclusion, the results of this sub-analysis of ASSURE trial focused on high-risk RCC patients underline the necessity of more selective criteria in the adjuvant setting, not only based on tumor staging but also on tumor biological and molecular features. This is absolutely required in order to carry the adjuvant approach for RCC patients into the era of personalized and precision medicine.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Peng Zhang, MD, PhD (Department of Urology, Zhongnan Hospital of Wuhan University, Wuhan, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.01.06). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Haas NB, Manola J, Dutcher JP, et al. Adjuvant Treatment for High-Risk Clear Cell Renal Cancer: Updated Results of a High-Risk Subset of the ASSURE Randomized Trial. JAMA Oncol 2017;3:1249-52. [Crossref] [PubMed]
- Haas NB, Manola J, Uzzo RG, et al. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): a double-blind, placebo-controlled, randomised, phase 3 trial. Lancet 2016;387:2008-16. [Crossref] [PubMed]
- Ravaud A, Motzer RJ, Pandha HS, et al. S-TRAC Investigators. Adjuvant sunitinib in high-risk renal-cell carcinoma after nephrectomy. N Engl J Med 2016;375:2246-54. [Crossref] [PubMed]
- Iacovelli R, Cossu Rocca M, Galli L, et al. Clinical outcome of patients who reduced sunitinib or pazopanib during first-line treatment for advanced kidney cancer. Urol Oncol 2017;35:541.e7-541.e13. [Crossref] [PubMed]
- Santoni M, Conti A, Porta C, et al. Sunitinib, pazopanib or sorafenib for the treatment of patients with late-relapsing (>5 years) metastatic renal cell carcinoma. J Urol 2015;193:41-7. [Crossref] [PubMed]
- Santoni M, Conti A, Procopio G, et al. Bone metastases in patients with metastatic renal cell carcinoma: are they always associated with poor prognosis? J Exp Clin Cancer Res 2015;34:10. [Crossref] [PubMed]
- Santoni M, Buti S, Conti A, et al. Prognostic significance of host immune status in patients with late relapsing renal cell carcinoma treated with targeted therapy. Target Oncol 2015;10:517-22. [Crossref] [PubMed]
- Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med 2015;373:1803-13. [Crossref] [PubMed]
- Hammers HJ, Plimack ER, Infante JR, et al. Safety and efficacy of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma: The checkMate 016 study. J Clin Oncol 2017;35:3851-8. [Crossref] [PubMed]
- Charlton JJ, Chatzidakis I, Tsoukatou D, et al. Programmed death-1 shapes memory phenotype CD8 T cell subsets in a cell-intrinsic manner. J Immunol 2013;190:6104-14. [Crossref] [PubMed]