
Icotinib improves progression free survival in epidermal growth factor receptor positive non-small cell lung cancer patients
Lung cancer, as defined by the National Cancer Institute, is the uncontrolled proliferation of cells lining the air passages and tissues of the lungs (1). Lung cancer is the second most common cancer in both men and women (with prostate and breast as the most common in men and women, respectively). It is estimated that lung cancer accounts for about 14% of all new cancer cases. The term ‘lung cancer’ is a broad term used to encompass the multitude of well-known histological subcategories such as lung adenocarcinoma, squamous cell carcinoma, large cell carcinoma, and small cell lung carcinoma (2). Lung adenocarcinoma is the most aggressive and prevalent type, contributing to about 45% of all lung cancer cases, followed by squamous cell carcinoma (25%), large cell carcinoma (10%), and small cell lung carcinoma (20%) (2-4).
Recent estimates on lung cancer are quite alarming. The American Cancer Society estimates that about 234,030 new cases of lung cancer will be diagnosed in the United States in 2018, with a greater prevalence in men as compared to women (121,680 and 112,350 respectively) (5). Moreover, about 154,050 deaths from lung cancer are predicted this year alone (83,550 in men and 70,500 in women) (5). Lung cancer is more common in the elderly population of 65 years and older, in fact, less than 2% of lung cancer patients are in the age population of 45 years or younger. Each year, about 1 in every 4 cancer related deaths are lung cancers (5). The mortality rate of lung cancer is higher than the combined rate of colon, breast, and prostate cancers. Undoubtedly, lung cancer continues to be the leading cause of cancer related deaths in both men and women (5).
Based on the morphology of cells, lung cancer can be classified into two subtypes; small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC). SCLC is more aggressive and fast growing cancer type that starts in the lung tissue and migrates to other parts of the body. SCLC accounts for 10% to 15% for all lung cancer cases (6). When observed under a microscope, the cells look small and oval shaped. NSCLC, on the other hand, is less aggressive and accounts for about 80% to 85% of all lung cancer cases (1,6). NSCLC may originate in squamous cells (squamous cell carcinoma), in cells lining the alveoli (adenocarcinoma), or in other large cells (large cell carcinoma) (4,7). It is pertinent for clinicians to understand this distinction for a more careful selection of chemotherapeutic agents in advanced stages of NSCLC, with or without a driver mutation. For example, patients with lung adenocarcinoma respond better to icotinib as compared to pemetrexed therapy and titrating the toxicity of bevacizumab in patients with squamous cell carcinoma (8). The growing use of the term ‘theranostics’ wherein, therapeutic and diagnostic strategies are meaningfully combined to develop targeted therapy, has now become a commonplace in NSCLC treatment. In recent years, researchers have used genomic sequencing to identify specific biomarkers, epigenetic mutations, tumor-suppressor/activator-genes that are potential targets for chemotherapy. The identification of mutant forms of epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) rearrangements in NSCLC progression has helped in the development of targeted therapy (9,10).
The EGFR, abbreviated as EGFR, ErbB1, or HER1, is a family of receptor tyrosine kinases that activate and elicit cell-signaling pathways leading to cell growth and survival (11,12). The PI3K-AKT-mTOR, MAPK and JAK/STAT pathways are amongst the most common pathways regulated by EGFRs (13). It has been reported that EGFR overexpression accounts for 40–80% of NSCLC cases thus, making it a promising target for chemotherapeutic drug development (1). However, it was subsequently discovered that the mutant forms of EGFR are more aggressive and resistant to conventional chemotherapeutic agents thus demanding development of new strategies to target the EGFR mutant-positive NSCLC. The mutation in exons 19 and 21 are the most common EGFR mutations (11,13). The exon 19 mutations are the in-frame deletion mutations that occur in the LREA motif (amino acids L747 to A750) and account for about 60% of all EGFR mutant-positive NSCLC cases. On the other hand, mutations on exon 21, wherein leucine is replaced by arginine thus, referred to as L858, account for about 35–40% of all EGFR mutant-positive NSCLC cases.
The first EGFR mutant-positive NSCLC case was reported in 2004 and since then numerous efforts have been made to develop small molecule tyrosine kinase inhibitors (TKIs) such as gefitinib (Iressa), erlotinib (Tarceva), and afatinib (Gilotrif), and monoclonal antibodies such as cetuximab (Erbitux) (14). For the treatment of EGFR mutant-positive NSCLC, the recommended first-line therapy includes one of three approved TKIs: gefitinib 250 mg p.o. daily, erlotinib 150 mg p.o. daily, or afatinib 40 mg p.o. daily (15). Both gefitinib and erlotinib inhibits the adenosine triphosphate (ATP) in a reversible and competitive manner. This ATP provides energy for the activation of EGFR, causing blockade of downstream signaling molecules. Contrastingly, afatinib is an irreversible inhibitor of both wild-type and mutant EGFR. The classical Iressa Pan-Asia Study (IPASS) of 2009 involving 1,217 patients from most of the East Asian countries with untreated stage IIIB or IV adenocarcinoma compared gefitinib or erlotinib versus carboplatin and paclitaxel chemotherapy for EGFR mutant-positive NSCLC (16). Patients receiving gefitinib or erlotinib had higher overall response rates (ORRs), better quality of life (QoL), and better progression-free survival (PFS) and overall survival (OS) compared to those receiving carboplatin and paclitaxel chemotherapy. These findings were further confirmed by a number of trials, to mention a few, OPTIMAL [2011], First-SIGNAL [2012], LUX-Lung 3 [2013], and LUX-Lung 6 [2014] (17-20). In each of these trials, the EGFR inhibitor gefitinib, erlotinib, or afatinib were compared to conventional chemotherapeutic agents, specifically in EGFR-mutant positive NSCLC. The results showed that the first-line EGFR TKIs exhibited superior ORRs, QoL, and PFS as compared to chemotherapy. Thus, it is important to identify the presence of EGFR mutations in NSCLC patients to select and implement a strict therapeutic regimen, consisting of the proper EGFR TKIs.
Icotinib, an orally available selective EGFR TKI, is used for first-line treatment of locally advanced or metastatic NSCLC or second or third line treatment for patients who failed to respond to at least one prior platinum-based chemotherapy (21,22). In 2011, the China Food and Drug Administration (CFDA) granted approval for the marketing of icotinib hydrochloride (23). Currently in the US, icotinib is being investigated in Phase IV clinical trials (22). In order to investigate icotinib’s inhibitory activity in EGFR mutation-positive lung adenocarcinoma, Shi and colleagues conducted a phase III open labeled study comparing icotinib versus cisplatin plus pemetrexed as maintenance therapy (24). Figure 1 explains the mechanism of action of icotinib.
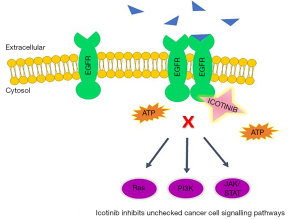
The current study, published in Annals of Oncology, assessed the efficacy and safety-profile of icotinib and compared it to the conventional platinum-based chemotherapy. A cohort of 296 patients with stage III or IV lung adenocarcinoma and an EGFR mutation-positive genotype was selected for this study. Exclusion criteria included patients less than 18 years old, any history of chemotherapeutic drug intake, patients who tested negative for EGFR mutation, or a patient history of renal, lung or cardiac disease. The patients were assigned to receive either icotinib or chemotherapy. Icotinib was administered p.o 125 mg, thrice daily while 75 mg/m2 cisplatin plus pemetrexed was administered i.v. for a 3-week cycle. The results showed that icotinib had a significantly higher PFS and better safety profile as compared to the first-line chemotherapy. The OS was found to be similar in both the therapeutic regimens. Interestingly, patients carrying exon 19-mediated EGFR mutations showed slightly higher PFS as compared to those carrying exon 21-mediated EGFR mutations, though this increase was not statistically significant. Furthermore, the interstitial lung disease, caused by icotinib, was found to be prevalent in individuals who were administered a minimum of one chemotherapy dose. Shi and colleagues have successfully highlighted the strength of this study by justifying their selection of cisplatin and pemetrexed chemotherapy as a control for the study. In summary, this phase III open labeled study has shown that icotinib alone as a first-line therapy improves the survival of EGFR mutation-positive lung adenocarcinoma patients with fewer side effects, as compared to cisplatin/pemetrexed plus pemetrexed maintenance therapy.
Though we found the results of the study promising for the prognosis of NSCLC patients, with fewer side effects, the study left some questions. First and foremost, the study design compared the treatment of icotinib, a small molecule tyrosine kinase inhibitor, versus chemotherapy treatment, a combination of alkylating agent (cisplatin) and folate antimetabolite (pemetrexed) chemotherapies. Though all treatments are considered anti-cancer agents, it is important to note the unrelated mechanisms of action between the two study arms. The study reveals the success of icotinib alone in improving survival of EGFR mutation-positive lung adenocarcinoma patients with fewer side effects, as compared with cisplatin/pemetrexed, however we are not able to accurately evaluate this success without having comparative treatment groups alongside using of another EGFR TKI. The statistical analysis section of the article states the progression free survival (11 months) on icotinib therapy was based on the data from gefitinib and erlotinib, yet the study did not include either of these currently approved EGFR TKI as comparative treatment group arms.
In addition, the study states while the dose of chemotherapy will be reduced if necessary, the reductions in the doses of icotinib (125 mg taken 3 times per day) were not mentioned. Moreover, the study states the regular dosing schedule will be interrupted if adverse events of grades 3 or 4 occur. Furthermore, we are told that the patients who did not recover to either grade 1 or 2 of adverse event (AE) will be pulled out of the study but it is not clear the percentage of patients who needed to temporarily cease treatment due to grade 3 or 4 AEs. Moreover, it is unclear that how those patients factored into the PFS statistics. Looking further into the AEs, results from the study indicated that the most common drug-related adverse effects were rash for the icotinib treated group, versus hematologic and gastrointestinal toxicities for the chemotherapy treated group. No description or insight into characterization, localization, or severity of the icotinib-related rash was provided.
Originally screening 669 patients over the course of 19 months, the study population of 296 having an EGFR mutation were included and distributed to either arm of the study. However, upon start of the study, it is mentioned that 11 patients on the platinum chemotherapy side were not given any treatment; as a result, 285 patients remained in the study. One hundred and forty-eight of these received icotinib while 137 were given chemotherapy. It is not clear why these two arms were not re-randomized to maintain equality in population numbers in both treatment arms. Nonetheless, the population size of the study was of relatively good size. Overall, the study reveals great promise for NSCLC patients, demonstrating icotinib as a first-line treatment option with relatively lower side effects as compared to chemotherapy.
Studies done in our lab showed for the first time that the up-regulation of thymidylate synthase (TS) in ABCG2-overexpressing cell line NCI-H460/MX20 plays a major role in resistance to pemetrexed therapy (21). We found that icotinib alone could significantly decrease the expression of TS which may be due to a reduction in E2F-1 expression (25). Moreover, pemetrexed increased both TS and ABCG2 expression. However, TS and ABCG2 expression were not altered by icotinib alone or in combination with pemetrexed, leading to pemetrexed resistance in NCI-H460/MX20 cells and tumor xenografts. Overall, we suggested that the inhibition of TS and ABCG2 expressions might overcome pemetrexed resistance in ABCG2-overexpressing NSCLC patients.
Acknowledgments
We thank Zhejiang Beta Pharma Inc. for providing the Icotinib hydrochloride tablets. We thank the Guangzhou Postdoctoral Foundation of International Training for Qingbin Cui from Guangzhou Medical University.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Wei Xu, MD (Division of Respiratory Disease, Department of Geriatrics, the First Affiliated Hospital of Nanjing Medical University, Nanjing, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.01.15). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shea M, Costa DB, Rangachari D. Management of advanced non-small cell lung cancers with known mutations or rearrangements: latest evidence and treatment approaches. Ther Adv Respir Dis 2016;10:113-29. [Crossref] [PubMed]
- Pirker R, Filipits M. Alectinib in RET-rearranged non-small cell lung cancer-Another progress in precision medicine? Transl Lung Cancer Res 2015;4:797-800. [PubMed]
- Gandara DR, Hammerman PS, Sos ML, et al. Squamous cell lung cancer: from tumor genomics to cancer therapeutics. Clin Cancer Res 2015;21:2236-43. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature 2014;511:543-50. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Chan BA, Coward JI. Chemotherapy advances in small-cell lung cancer. J Thorac Dis 2013;5:S565-78. [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature 2012;489:519-25. [Crossref] [PubMed]
- Johnson DH, Fehrenbacher L, Novotny WF, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol 2004;22:2184-91. [Crossref] [PubMed]
- Ding L, Getz G, Wheeler DA, et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature 2008;455:1069-75. [Crossref] [PubMed]
- Pao W, Girard N. New driver mutations in non-small-cell lung cancer. Lancet Oncol 2011;12:175-80. [Crossref] [PubMed]
- Arteaga CL. The epidermal growth factor receptor: from mutant oncogene in nonhuman cancers to therapeutic target in human neoplasia. J Clin Oncol 2001;19:32S-40S. [PubMed]
- Kathawala RJ, Gupta P, Ashby CR Jr, et al. The modulation of ABC transporter-mediated multidrug resistance in cancer: a review of the past decade. Drug Resist Updat 2015;18:1-17. [Crossref] [PubMed]
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2001;2:127-37. [Crossref] [PubMed]
- Politi K, Herbst RS. Lung cancer in the era of precision medicine. Clin Cancer Res 2015;21:2213-20. [Crossref] [PubMed]
- Yamaoka T, Ohba M, Ohmori T. Molecular-targeted therapies for epidermal growth factor receptor and its resistance mechanisms. Int J Mol Sci 2017;18:E2420 [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [Crossref] [PubMed]
- Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:213-22. [Crossref] [PubMed]
- Han JY, Park K, Kim SW, et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol 2012;30:1122-8. [Crossref] [PubMed]
- Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327-34. [Crossref] [PubMed]
- Wang DS, Patel A, Shukla S, et al. Icotinib antagonizes ABCG2-mediated multidrug resistance, but not the pemetrexed resistance mediated by thymidylate synthase and ABCG2. Oncotarget 2014;5:4529-42. [Crossref] [PubMed]
- Tan F, Shi Y, Wang Y, et al. Icotinib, a selective EGF receptor tyrosine kinase inhibitor, for the treatment of non-small-cell lung cancer. Future Oncol 2015;11:385-97. [Crossref] [PubMed]
- Guan YS, He Q, Li M. Icotinib: activity and clinical application in Chinese patients with lung cancer. Expert Opin Pharmacother 2014;15:717-28. [Crossref] [PubMed]
- Shi YK, Wang L, Han BH, et al. First-line icotinib versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for patients with advanced EGFR mutation-positive lung adenocarcinoma (CONVINCE): a phase 3, open-label, randomized study. Ann Oncol 2017;28:2443-50. [Crossref] [PubMed]
- Yeh KH, Cheng AL, Wan JP, et al. Down-regulation of thymidylate synthase expression and its steady-state mRNA by oxaliplatin in colon cancer cells. Anticancer Drugs 2004;15:371-6. [Crossref] [PubMed]


