
Is durvalumab the solution for unresectable stage III non-small cell lung cancer?
Lung cancer is the leading cause of cancer deaths in the United States and worldwide (1). The majority of patients with newly diagnosed non-small cell lung cancers (NSCLC) have metastasis at presentation but a large group of patients present with locally or regionally advanced disease. Stage III NSCLC represents a broad range of patients ranging from a microscopic focus of disease in a single mediastinal lymph node to extensively involved contralateral lymph nodes. Management of stage III NSCLC is accordingly complex and subject to much debate. Concurrent chemoradiation therapy has been the preferred treatment of stage III NSCLC in an effort to treat both locoregional and micrometastatic disease. Although the definition of resectable stage III NSCLC is not uniformly applied, some patients who have clearing of a single mediastinal node with induction chemoradiation therapy may be later consider for surgery. Earlier studies examined sequential therapy (chemotherapy followed by radiotherapy) in order to improve tolerance, but these sequential approaches failed to improved patients’ outcomes (2). The superiority of concurrent chemoradiotherapy has been demonstrated by two large phase 3 clinical trials. In the Radiation Therapy Oncology Group (RTOG) 9410 trial, 610 patients with unresectable stage III disease were randomly assigned to two cycles of cisplatin plus vinblastine with concurrent or sequential radiotherapy (RT) (60 Gy in 30 fractions). Those receiving concurrent chemoradiotherapy experienced improvement in survival [17.0 vs. 14.6 months; hazard ratio (HR) for death 0.81, 95% CI, 0.663–0.996] (3). Similarly, in a Japanese study, 320 patients were treated with cisplatin, mitomycin, and vindesine and randomly assigned to concurrent versus subsequent radiation. Concurrent therapy was associated with improved response rate (84% vs. 66%) and median survival (17 vs. 13 months) (4).
Platinum based regimens are the most commonly used with concurrent radiotherapy. Cisplatin with etoposide and weekly carboplatin with paclitaxel demonstrated comparable overall survival (OS) in a phase 3 trial (5), and have been used interchangeable in clinical practice. Table 1 summarizes phase 3 trials in unresectable III NSCLC.
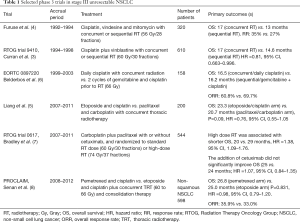
Full table
Unfortunately, the survival of patients with unresectable stage III NSCLC remains poor at approximately 8 months with only 15% of patients being alive at 5 years (9). The PACIFIC trial is the latest (and only) advance in the treatment of this subset of patients in the past decade (10).
Durvalumab (MEDI4736) is an IgG1 monoclonal antibody that binds to programmed cell death ligand 1 (PD-L1, aka B7-H1 and CD274) and blocks immunosuppressive interactions with programmed cell death 1 (PD-1, aka CD279) and CD80. Preclinical studies have demonstrated that the antitumor activity of durvalumab was potentiated with the cytotoxic chemotherapy oxaliplatin, suggesting a potential benefit from the combination of chemotherapy and PD-L1 inhibition (11).
Durvalumab was tested in a large phase 1/2 trial that studied its safety and clinical activity in patients with solid tumors including over 300 patients with NSCLC. Durvalumab (10 mg/kg) was administered intravenously every 2 weeks for up to 12 months or until disease progression. Overall durvalumab was well tolerated and 10% of patients experienced grade 3 or higher drug-related adverse events (AEs) most commonly including fatigue, hyponatremia, and colitis with 5% of patients discontinuing the drug due to adverse events, showing a similar AEs profile to other immune checkpoint inhibitors. Responses to treatment were more commonly seen in patients whose tumors had high levels of PD-L1 expression [25% overall response rate (ORR)] compared to those with low levels or absent expression (6% ORR). The OS for patients with PD-L1 expressing tumors was also impressive with mOS of 17.8 months in the second-line and 13 months in the third-line (12).
Durvalumab has also been studied in a phase 1/2 trial in patients with advanced urothelial bladder cancer; the ORR was 31% with higher response in the PD-L1 positive subgroup (46.4%). Durvalumab also demonstrated a manageable safety profile (13). These findings lead to the United States Food and Drug Administration (FDA) approval of durvalumab in locally advanced or metastatic urothelial carcinoma after progression on platinum-based chemotherapy.
Preclinical data suggest that chemoradiation may up-regulate the PD-L1 expression in tumor cells, which may correlate with durvalumab activity. The abscopal effect has generated a great deal of interest in combining immunotherapy and radiation. The abscopal effect refers to the response of a non-radiated lesion after radiation of another lesion. This effect has been seen in patients with melanoma treated with immunotherapy and radiation (14). The immune system is thought to mediate the abscopal effect specifically through PD-1 as some have demonstrated in murine models (15). There are many mechanisms of tumor immune escape that radiotherapy has the potential to overcome through the release of immunogenic private antigens, enhanced tumor expression of MHC-I, release of the TLR4 agonist HMGB-1, the generation of tumor specific cytotoxic T cells and increasing the expression of PD-L1 on tumor cells (15-17). In preclinical models the combination of radiotherapy and PD-1 blockade was described to be tumor-antigen specific, not dependent on specific tumor biology (which can partially explain some of the findings from the PACIFIC trial) and not limited to the host genetic backgrounds (15,17).
In the case of chemotherapy followed by immunotherapy, Zhang et al. demonstrated that paclitaxel, etoposide and 5-fluorouracil were able to induced PD-L1 surface expression in human breast cancer cells, which then promoted PD-L1-mediated T cell apoptosis, suggesting a potential link between chemotherapy and immunoresistance (18). Others have similarly demonstrated that various chemotherapies may upregulate PD-L1 expression by tumor cells (19). Up-regulation of tumor-associated PD-L1 by cytokines secondary to the effects of chemotherapy in the tumor microenvironment could also potentially contribute to this process and diminish anti-tumor immunity (18).
Durvalumab was studied in the PACIFIC trial, a multicenter, international, phase 3 study in patients with unresectable stage III NSCLC. Following completion of chemoradiation therapy, patients were randomly assigned within 1 to 42 days in a 2:1 ratio to receive consolidation therapy with durvalumab at a dose of 10 mg/kg or matching placebo every 2 weeks for up to 12 months. Eligible patients had received two or more cycles of platinum-based chemotherapy and concurrent definitive radiation (54 to 66 Gy). Patients with disease progression while receiving chemoradiation were excluded. The exclusion and inclusion criterion were standard for most immunotherapy trials.
Over 700 patients with unresectable stage III NSCLC were randomized to durvalumab or placebo. At a planned interim analysis, durvalumab compared to placebo improved median progression-free survival (PFS) (16.8 versus 5.6 months; HR for disease progression or death 0.52, 95% CI, 0.42–0.65), response rate [28% vs. 16%; relative risk (RR) 1.78, 95% CI, 1.27–2.51], and median time to death or distant metastasis (23.2 vs. 14.6 months; HR =0.52, 95% CI, 0.39–0.69). The benefit in PFS with durvalumab was observed irrespective of PD-L1 expression before chemoradiotherapy and was evident in both smokers and nonsmokers. Overall survival results were not mature at the time of publication. Grade 3 or 4 AEs were similar with the durvalumab (29.9%) and placebo group (26.1%). Additionally, patients that received radiation less than 14 days from randomization appeared to benefit more from durvalumab. No significant differences were observed across chemotherapy regimens (10).
Some have suggested that the placebo arm in the PACIFIC trial did poorly compared to previous studies with a median PFS of 5.6 months, whereas the median PFS for the control group in the RTOG 0617 trial was 11.8 months (7), and the median PFS was 8 months in the control group in the Japanese trial comparing concurrent versus sequential chemoradiation (4). Similarly in the Stimulating Targeted Antigenic Response to NSCLC (START) trial, in which randomization occurred after chemoradiotherapy, the PFS among patients in the control group was 8.4 months (20). Since the PFS was calculated from initiation of chemoradiotherapy in some of these other studies, but was calculated at the time of randomization in the PACIFIC trial, the performance of the control arm in PACIFIC was actually in line with prior reports.
Given the maturity of the PACIFIC trial with the published data to date, many questions remain including: will an OS benefit be observed? Could patients be re-challenged with a different immune-checkpoint inhibitor at the time of relapse? Can platinum-based regimens be used for the next line of therapy?
Patients with stage III NSCLC represent a very heterogeneous group, from a variety of histologic subtypes to differences in the extent of nodal involvement. The benefits of consolidative therapy with durvalumab can be applied to a specific group of patients with unresectable stage III NSCLC: those with good performance status (i.e., fit to receive definite chemoradiation), mean radiation dose to the lung less than 20 Gy (due to increased pneumonitis risk) and no disease progression while receiving concurrent chemoradiation. Despite being an international study, the trial failed to recruit minority patients. Only 14 (2%) black patients were recruited and data regarding the recruitment of Hispanic patients is lacking, putting into question the generalizability of these findings to all patients with unresectable stage III NSCLC. On the other hand, the benefits of durvalumab as consolidation therapy were observed in patients with squamous and non-squamous histology as well as smokers and non-smokers, irrespective of PD-L1 tumor expression. These findings support that consolidation therapy with durvalumab is broadly applicable to otherwise unselected patients with stage III NSCLC. The benefit of consolidative immunotherapy following chemoradiation regardless of baseline PD-L1 expression may be partially explained by the heterogeneity of PD-L1 expression in NSCLC (21,22) and the immunoregulation induced by chemoradiation, which could “level-up” all patients to the benefits of immunotherapy (17,18,23).
Based on the results of the interim analysis, the PACIFIC trial met its primary end-point, with a significant improvement in PFS (approximately 12 months) in the durvalumab arm compared to placebo, with benefit seen across all groups. The authors reported a benefit in the median time to death or distant metastasis, 23.2 months with durvalumab versus 14.6 months in the placebo group, with lower incidence of new brain metastasis with durvalumab (5.5% vs. 11%). Given that overall survival is the standard when it comes to evaluating outcomes in clinical trials these findings suggest that an improvement in OS will be observed with ongoing follow-up. In our perspective, the OS survival data are imperative to generate definite conclusions regarding durvalumab in unresectable stage III NSCLC, but we are very encouraged that durvalumab will become the new standard of care in this setting.
Patients with cancer are three times more likely to file for bankruptcy compare to patients with other chronic conditions (24), forcing patients to make life and death decisions based on their financial status. In the PACIFIC trial patients were placed on durvalumab for 12 months or until disease progression. With an acquisition cost of durvalumab around $15,000/month (25), this new regimen would potentially bring significant financial toxicity to patients once it is FDA approved or if it is currently being used off-label. Similarly, quality of life while on treatment plays a significant role in selecting therapies. The study team plans to compare patient-reported health-related quality of life between the placebo arm (which had an identical administration schedule) and the durvalumab arm (10). Results should be evaluated carefully, as the 12-month commitment is onerous.
Based on the results from the PACIFIC trial, durvalumab was granted priority review by the FDA for the treatment of patients with unresectable stage III NSCLC. Regarding the future of durvalumab, promising activity has been observed in combination with other immune checkpoint inhibitors, and platinum-based chemotherapy. At the time this editorial was written, over 100 clinical trials are reported as active and recruiting in clinicaltrials.gov studying durvalumab as monotherapy or in combination regimens.
In summary, the PACIFIC trial showed improved PFS with durvalumab as consolidation therapy in patients with unresectable stage III NSCLC after two cycles of chemotherapy and concurrent radiation when compared to placebo. While the OS data are not yet mature, a decreased frequency of distant metastasis (particularly brain metastasis) with durvalumab was also observed. Based on pre-clinical data, chemoradiation may play a role in the upregulation of PD-L1 expression which may correlate with durvalumab activity. Further clinical trials studying combination therapies, duration of treatment and timing of chemoradiation with or without surgery will help refine the role of durvalumab in the treatment of stage III unresectable NSCLC. Regardless, durvalumab consolidation therapy represents the most significant advance in the treatment of unresectable stage III NSCLC and may receive approval in the near future.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: TThis article was commissioned and reviewed by the Section Editor Runzhe Chen (Department of Thoracic/Head & Neck Medical Oncology, University of Texas MD Anderson Cancer Center, Houston, TX, USA).
Conflicts of Interest: Dr. Duma has no conflicts of interest to declare. Dr. Mansfield reports payments to his institution from AbbVie, Genentech, BMS and Trovagene for consulting, and payment to his institution from Novartis for research.
RT, radiotherapy; Gy, Gray; OS, overall survival; HR, hazard ratio; RR, response rate; RTOG, Radiation Therapy Oncology Group; NSCLC, non-small cell lung cancer; ORR, overall response rate; TRT, thoracic radiotherapy.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- O'Rourke N, Roque IFM, Farre Bernado N, et al. Concurrent chemoradiotherapy in non-small cell lung cancer. Cochrane Database Syst Rev 2010;CD002140 [PubMed]
- Curran WJ Jr, Paulus R, Langer CJ, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst 2011;103:1452-60. [Crossref] [PubMed]
- Furuse K, Fukuoka M, Kawahara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol 1999;17:2692-9. [Crossref] [PubMed]
- Liang J, Bi N, Wu S, et al. Etoposide and cisplatin versus paclitaxel and carboplatin with concurrent thoracic radiotherapy in unresectable stage III non-small cell lung cancer: a multicenter randomized phase III trial. Ann Oncol 2017;28:777-83. [PubMed]
- Belderbos J, Uitterhoeve L, van Zandwijk N, et al. Randomised trial of sequential versus concurrent chemo-radiotherapy in patients with inoperable non-small cell lung cancer (EORTC 08972-22973). Eur J Cancer 2007;43:114-21. [Crossref] [PubMed]
- Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol 2015;16:187-99. [Crossref] [PubMed]
- Senan S, Brade A, Wang LH, et al. PROCLAIM: Randomized Phase III trial of pemetrexed-cisplatin or etoposide-cisplatin plus thoracic radiation therapy followed by consolidation chemotherapy in locally advanced nonsquamous non-small-cell lung cancer. J Clin Oncol 2016;34:953-62. [Crossref] [PubMed]
- Yoon SM, Shaikh T, Hallman M. Therapeutic management options for stage III non-small cell lung cancer. World J Clin Oncol 2017;8:1-20. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage iii non-small-cell lung cancer. N Engl J Med 2017;377:1919-29. [Crossref] [PubMed]
- Stewart R, Morrow M, Hammond SA, et al. Identification and Characterization of MEDI4736, an Antagonistic Anti-PD-L1 Monoclonal Antibody. Cancer Immunol Res 2015;3:1052-62. [Crossref] [PubMed]
- Antonia SJ, Brahmer JR, Khleif S, et al. Phase 1/2 study of the safety and clinical activity of durvalumab in patients with non-small cell lung cancer (NSCLC). Ann Oncol 2016;27:1216PD.
- Massard C, Gordon MS, Sharma S, et al. Safety and Efficacy of Durvalumab (MEDI4736), an anti-programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J Clin Oncol 2016;34:3119-25. [Crossref] [PubMed]
- Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012;366:925-31. [Crossref] [PubMed]
- Park SS, Dong H, Liu X, et al. PD-1 Restrains Radiotherapy-Induced Abscopal Effect. Cancer Immunol Res 2015;3:610-9. [Crossref] [PubMed]
- Kalbasi A, June CH, Haas N, et al. Radiation and immunotherapy: a synergistic combination. J Clin Invest 2013;123:2756-63. [Crossref] [PubMed]
- Mansfield AS, Park SS, Dong H. Synergy of cancer immunotherapy and radiotherapy. Aging (Albany NY) 2015;7:144-5. [Crossref] [PubMed]
- Zhang P, Su DM, Liang M, et al. Chemopreventive agents induce programmed death-1-ligand 1 (PD-L1) surface expression in breast cancer cells and promote PD-L1-mediated T cell apoptosis. Mol Immunol 2008;45:1470-6. [Crossref] [PubMed]
- Terra SBSP, Mansfield AS, Dong H, et al. Temporal and spatial heterogeneity of programmed cell death 1-Ligand 1 expression in malignant mesothelioma. Oncoimmunology 2017;6:e1356146 [Crossref] [PubMed]
- Butts C, Socinski MA, Mitchell PL, et al. Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): a randomised, double-blind, phase 3 trial. Lancet Oncol 2014;15:59-68. [Crossref] [PubMed]
- Mansfield AS, Aubry MC, Moser JC, et al. Temporal and spatial discordance of programmed cell death-ligand 1 expression and lymphocyte tumor infiltration between paired primary lesions and brain metastases in lung cancer. Ann Oncol 2016;27:1953-8. [Crossref] [PubMed]
- Mansfield AS, Murphy SJ, Peikert T, et al. Heterogeneity of Programmed Cell Death Ligand 1 Expression in Multifocal Lung Cancer. Clin Cancer Res 2016;22:2177-82. [Crossref] [PubMed]
- Cheng M, Durm G, Hanna N, et al. Can radiotherapy potentiate the effectiveness of immune checkpoint inhibitors in lung cancer? Future Oncol 2017;13:2503-5. [Crossref] [PubMed]
- Ramsey SD, Fedorenko CR, Snell KS, et al. Cancer diagnosis as a risk factor for personal bankruptcy. J Clin Oncol 2011;29:6007. [Crossref]
- Reuters. BRIEF-AstraZeneca's durvalumab average monthly cost around $15,000. Reuters.com. 2017. Available online: https://www.reuters.com/article/brief-astrazenecas-durvalumab-average-mo/brief-astrazenecas-durvalumab-average-monthly-cost-around-15000-idUSL8N1I32KY


