
Hepatocellular carcinoma: the rising tide from east to west—a review of epidemiology, screening and tumor markers
Introduction
More than half a million individuals per year are diagnosed with hepatocellular carcinoma (HCC). From a global perspective it ranks as the fifth most common cancer in men and the seventh in women (1). Worldwide it ranks third in cancer mortality behind lung and gastric cancer. In the present decade, we are experiencing a shift in incidence that is declining in the Asian-Pacific region, where it is primarily due to vertical transmission of hepatitis B, to an increasing incidence in the Western world due to the maturation of the hepatitis C epidemic. In the U.S. the increase is three fold in the last decade (2). If we understand the epidemiology and risk factors and screen accordingly we can make an impact.
Epidemiology of HCC
Geographic variation in the incidence of HCC for the most part is dependent upon its primary risk factors.
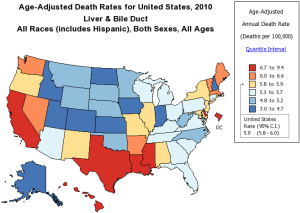
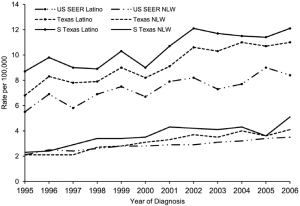
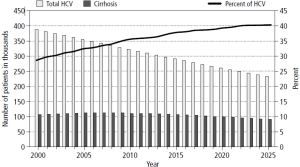
In Eastern Asia and sub-Sahara Africa where the highest incidence rates occur (>20/100,000), hepatitis B infection, mostly from vertical transmission, accounts for 70% of the HCC cases. In Europe and North America, considered low incidence areas (<5/100,000), hepatitis C and alcohol account for 50-60% and 10-20% of the HCC cases respectively. The incidence rates of HCC are decreasing in some areas in China primarily in Hong Kong, Shanghai, and Singapore related to universal vaccination for hepatitis B (3-6). HCC in Japan, where hepatitis C is the most common etiology, is also experiencing a decreasing incidence. This is secondary to the timing of acquisition where most infections were through blood transfusions 20 years prior to the US epidemic in which IV drug experimentation predominated. HCC incidence in the United States and Europe is increasing. The driving force is predominantly hepatitis C infection. In the U.S., the overall age adjusted incidence and mortality rates tripled between 1975 and 2007 (1). The annual percentage increase was 4.3% per year. HCC has become the fastest rising cause of cancer related death in the United States. There are pockets in the United States were the mortality of HCC is high (Figure 1) (7). These areas include Texas, Louisiana, and Mississippi. In the U.S., the age adjusted HCC incidence for Asian/Pacific Islanders is three times that of Caucasians. However, the annual percentage increase in HCC per ethnicity in the U.S. between 1992 and 2005 was highest among American Indians and Alaskan natives at 5.0%. Black, white, and Hispanics had an annual percentage increase of 4.9%, 4.6%, and 4.0% respectively (8). Regional differences in the incidence of HCC also exist. The HCC incidence rate among South Texas Latino men and women (17.3/100,000 and 5.4/100,000) is 45% and 42% higher than the incidence of HCC in a comparative U. S. SEER Latino population (9) (Figure 2). This may reflect differences in the incidence of hepatitis C, diabetes, and alcohol consumption. Increasing HCC incidence is linked to increasing cirrhosis. Although noncirrhotic hepatitis B is notoriously linked to HCC, 70-80% of hepatitis B HCC occurs in cirrhosis patients. In hepatitis C, greater than 90% of HCC patients have cirrhosis. In Texas 30-35% of hepatitis C virus (HCV) patients presenting for the first time to the physician’s office will have established cirrhosis (10) (Figure 3). This is projected to increase over the next decade. The prevalence of cirrhosis and decompensated liver disease in U.S. veterans doubled between 1996 and 2006. In this same study, the prevalence of HCC increased ten times.
Foreign born persons accounted for almost a third of the U.S. HCC diagnosis between 2000 and 2005. Eighty percent of Asian-Pacific Islanders and 40% of Hispanics HCC patients were born outside the United States during this time period. Foreign born Caucasians accounted for 17%, blacks for 6%, and American Indians/Alaskan Natives 3% (11).
It is rare for HCC to occur before the age of 40. Female rates peak five years older than the peak age group for males (12). In the U.S. between 2000 and 2005 the majority of the increased incidence of HCC occurred among men age 52-59 years (8). In the U.S. it is becoming more common for HCC to occur in a younger population. The driving factor behind this is a maturation of the hepatitis C epidemic.
Risk factors for HCC
Risk factors for HCC can be divided into modifiable and non-modifiable risk factors (Table 1). Unfortunately, we cannot change our age, gender or race; however, we do have the ability to modify risk factors such as hepatitis C, hepatitis B, HIV, obesity, diabetes, alcohol intake and environmental exposures.
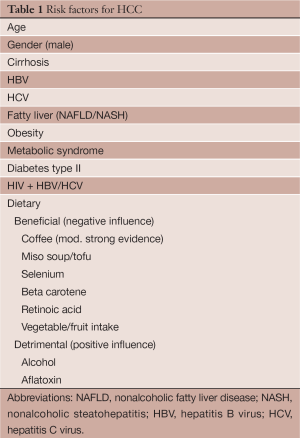
Full table
Gender
HCC occurs more often in males (ratio: 2:1 to 4:1) (13). This may reflect that men are more likely to be infected with viral hepatitis, consume alcohol, smoke cigarettes, and have a higher body mass index than women. Gender differences do exist in the liver with the masculinization or feminization occurring early in development peaking at puberty. These sexual differences involve gender specific gene expression, mitochondrial function, microsomal enzyme activity, membrane lipid composition and immune responses (14-16). Higher testosterone levels play a role. Elevated testosterone levels or the intake of anabolic steroids have been associated with increased incidence of liver adenomas and HCC. Higher serum levels of testosterone have been linked to HCC risk in nested case control studies of hepatitis B virus (HBV) carriers in Taiwan and Shanghai (17). High testosterone levels have been linked to advanced hepatic fibrosis and inflammation in males with chronic hepatitis C infection (18). In animal models androgens have been shown to increase the transcription of hepatitis B genes and bind directly to the viral genome sites (19). Gender disparity in IL6 production may also play a role. IL6 is a known cytokine associated with inflammation and implicated in modeling cell growth. IL6 is increased in HCC patients. Male dominant HCC incidence disappeared when production was blocked in mice (20).
Estrogens may play a role in the incidence of HCC among women. Cases control studies have shown a 5-fold increase in HCC in women with more than five years exposure to oral contraceptives (21-23).
Hepatitis B
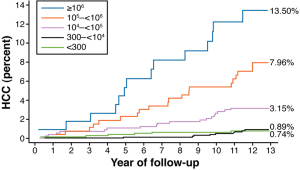
Hepatitis B accounts for over 50% of HCC incidence worldwide (24). In endemic areas the transmission is most often vertical (mother to child). Here, the timing of the infection is early thus, the occurrence of HCC appears earlier. Most cases of hepatitis B related HCC occur in patients with cirrhosis. The lifetime relative risk for HCC is 15-20 times greater in HBsAg positive individuals compared to HBsAg negative individuals (25). The individual lifetime risk for persons with chronic hepatitis B infection is between 10% and 25% (26). High viral load, genotype (C in Asia, D in North America), longer duration infection, and co-infection with hepatitis C, HIV, or HDV, increase the risk of HCC (27,28). Active viral replication (HBeAg positivity) confers an increased HCC risk 60 times verses only 10 times in non-replicating HBsAg men. HBV-DNA levels have been correlated in a dose response relationship to the later development of HCC in patients followed for a mean of 11.4 years. The hazard ratio of developing HCC was found to be 1.1 for participants with serum of HBV DNA levels of 300 to 9,999 copies/mL, 2.3 for 10,000 to 99,999 copies/mL, 6.6 for 100,000-999,999 copies/mL and 6.1 for 1 million copies/mL or greater (27,29) (Figure 4). This dose dependent correlation became increasingly stronger in a stepwise analysis sequentially removing patients with elevated serum ALT levels, seropositivity for HBeAg, and cirrhosis. A significant difference is seen in cumulative incidence of HCC in patients whose HBV DNA levels were >10,000 copies/mL (3.75 vs. 1.37) although the greatest difference occurred with >100,000 copies/mL (12,17). The cumulative incidence of HCC ranged from 1.3% with undetectable levels of HBV-DNA to 14.9% with >100,000 copies/mL. Additionally, in a study with follow up of 11 years, the relative risk of mortality from HCC was not significant for low viral load persons (HBV DNA less than 100,000 copies/mL) versus 10.7 with high viral load (HBV DNA >100,000 copies/mL) (30). It is unclear if these observations can be carried over in the Western world where the majority of hepatitis B viral infections occur horizontally. This correlation may not occur in infected persons <30 yrs who may be immune tolerant.
Alcohol consumption increases the risk of HCC in hepatitis B patients. Moderate drinking (≥3 drinks per week for >15 years) increases the odds ratio of HCC 3 to 4 fold (31). HBV-HCC occurred approximately ten years younger in chronic alcohol users (32,33).
Antiviral treatment reducing the levels of hepatitis B viral DNA can affect HCC occurrence. The cumulative HCC incidence at five years was reduced to 3.7% in an entecavir treated group versus 13.7% in controls. The reduction in HCC was greatest in those patients at high-risk for HCC especially in those with cirrhosis. Entecavir is superior to lamivudine in reducing the incidence of HCC (34).
Hepatitis C
The risk of HCC is increased 17-20 fold in HCV infected persons versus HCV negative controls (35). HCC mostly develops in cirrhotic individuals although in the HALT-C trial 8% occurred in patients with advanced fibrosis (36). Once cirrhosis occurs, the incidence of HCC is 1-4% per year.
The global incidence of hepatitis C is approximately 2% (37). In the U.S., between 4.1 and 5 million persons have antibodies to HCV and 3.2 to 3.4 million persons are chronically infected (38). The “biologic clock” (infection to cirrhosis to HCC) for hepatitis C is dependent on the time of infection. The average time from inoculation of HCV virus to the development of cirrhosis is 24 years and to the development of HCC 29 years. In Japan, most infections occurred in the 1920s and 1940s from a contaminated blood supply while in North America most infectious occurred between 1960s and 1970s, largely as a result of intravenous drug experimentation. Thus, the incidence of HCC has peaked in Japan and is declining. In the U.S. the incidence is increasing and is expected to peak within the next five years (39,40). HCV was present in the blood supplies in North America until the development of screening tests in 1990 resulted in a dramatic decline in this infection route. HCC incident rates of 1 per 1,000 persons/year have been linked to recipients of HCV contaminated blood or blood products. Unlike hepatitis B, HCC risk association with viral factors such as genotype and viral load are less important than host factors such as time of acquisition, male gender, alcohol intake, metabolic syndrome, diabetes or co-infection with HIV/HBV. Co-infection with HBV dramatically increases occurrence of HCC with an odds ratio of 165 versus 17 for hepatitis C and 23 for HBV alone (41). The consumption of heavy alcohol (>50 g/day) results in increased incidence and earlier development of HCC. This synergistic increase is between 1.7 and 2.9 fold when compared to HCV-HCC alone (42).
Treatment resulting in a sustained viral response (SVR) results in a reduction of the incidence of HCC. A SVR is associated with 54% reduction in all-cause mortality (43). The reduction in HCC with a SVR occurs whether or not the patient has advanced fibrosis or cirrhosis. In persons at all stages of liver disease HCC occurred in 1.5% responding to treatment compared to 6.2% who did not respond. In patients with advanced liver disease the reduction of risk was similar but HCC occurred in 4.2% of SVR responders in contrast to 17.8% of nonresponders.
Importantly, even with the achievement of a SVR a risk for the development of HCC remains.
Nonalcoholic fatty liver disease (NAFLD)
In developed countries, the most common form of liver disease is NAFLD (44). Being overweight and obesity is associated with a higher risk of HCC. Men with a body mass index over 35 kg/m2 are four times more likely to die from liver cancer when compared to a control group with normal body mass index (18.5-24.9 kg/m2) (45). The relative risk of HCC is 117% for overweight subjects and 189% for obese patients (46). In the United States, 30% of the general population and 90% of the morbidly obese have fatty liver disease. The inflammatory component of NAFLD, nonalcoholic steatohepatitis (NASH), is estimated to be present in 5-7% of these patients (47). This may be an underestimate as reflected by a study in San Antonio, Texas which demonstrated NASH in 31% of patients who were found to have fatty liver by ultrasound (US) criteria (48).
Type II diabetes, often a component of fatty liver and the metabolic syndrome increases the risk of hepatocellular cancer threefold (49). Type II diabetes is associated with a hazard rate ratio of 2.16 for HCC (50). NAFLD is present in 74% of type II diabetics on liver biopsy. In a large healthcare database study between 2002 and 2008, HCC-NAFLD/NASH was found to be the most common underlying etiologic risk factor (59%), followed by diabetes (36%) and HCV infection (22%) (51). A European study analyzing HCC cases identified steatohepatitis as the leading etiology in 24% compared to chronic HCV in 23.3%, chronic HBV 19.3%, and alcoholic liver disease 12.7% (52).
The majority of HCC-NAFLD occurs in men (53). Compared to women, men develop HCC with less fibrosis and cirrhosis. The mean age at presentation is 70 years. The risk for HCC in NAFLD is less than that of hepatitis C. The cumulative U.S. incidence of HCC in NASH patients has been shown to be 2.6% compared to 4.0% with HCV. In Japan, the cumulative incidence was 11.3% for HCV versus 30.5% for NAFLD related cirrhosis.
HCC has increasingly been reported in the non-cirrhotic NAFLD. Concurrent metabolic syndrome and steatohepatitis has been found as risk factors in these patients. Non cirrhotic HCC was reported in 116 NAFLD patients from 2004 to 2012 representing one third of all cases. In a Japanese cohort which included 87 cases of HCC nearly half were found not to have cirrhosis. HCC has also been reported in NAFLD patients with neither steatohepatitis nor fibrosis.
A large proportion of cryptogenic cirrhosis is secondary to NAFLD (54-56). This is supported by a significant prevalence of diabetes and obesity in these patients. Patients with cryptogenic cirrhosis frequently develop NAFLD and NASH post transplantation. Cryptogenic cirrhosis accounts for up to one quarter of HCC cases. Retrospective reviews have correlated the occurrence of diabetes, insulin resistance and dyslipidemia, elements of the metabolic syndrome and NAFLD, in many of these patients.
Diabetes
Diabetes is an independent risk factor for HCC (57,58). Diabetics have been shown to have between a 1.8 and 4 fold increased risk. Although closely associated with obesity and NAFLD, the risk of HCC remains after excluding patients with hepatitis C, hepatitis B, alcohol use and fatty liver disease. A graded dose response between fasting blood glucose and HCC risk has been reported that was independent of BMI (59). The risk is equally present in males and females. This association has been shown to be stronger in studies with a follow up period of >6 years. This increased risk is most evident in type II diabetics.
Dietary factors
Alcohol
Alcohol abuse in the United States occurs in more than 18 million people. The prevalence rate is five times higher than hepatitis C (60). In Europe, alcohol abuse accounts for 40-50% of all HCC cases (61). Alcohol consumption in 11-15-year-old has increased by two thirds since 1980 in the United Kingdom.
HCC risk in alcoholics is mostly associated with cirrhosis. Once decompensated cirrhosis develops risk is approximately 1%/yr. The risk increases with daily alcohol intake. An Italian study showed the risk was negligible for those who drink <40 g/d (one drink =12-14 grams). However, risk increases 1.5 (0.7-2.9) times for ingesting between 40-80 g/d and 7.3 (4.0-13.1) for those drinking >80 g/d (62). The risk of HCC increases above 1 when daily ethanol consumption exceeds 60 g per day, increasing in a linear fashion thereafter (62). In the U.S., patients reporting drinking any alcohol versus total abstinence had an adjusted odds ratio for HCC of 2.4. This rose to 4.5 for drinking >80 g/day. Discontinuing alcohol once cirrhosis occurs does not seem to lower the incidence of HCC. Alcohol when combined with HBV and HCV acts synergistically increasing the incidence of HCC 2-4 fold (63).
Aflatoxin
Aflatoxins are naturally occurring compounds produced by Aspergillum species (molds) that grow on grains, corn, peanuts, or soybeans stored in warm humid conditions (64). Aflatoxin B1 is a potent hepatocarcinogen producing neoplasms in rodents and primates. The risk of HCC is dependent on dose and duration of exposure. The metabolite AFB-1 binds to DNA and produces a mutation in the p53 tumor suppressor gene. Aflatoxin exposure is more prevalent in rural areas. It has a synergistic effect on hepatitis B and C induced liver cancer. The risk of liver cancer is 30 times greater with chronic HBV plus aflatoxin exposure than with aflatoxin exposure alone (65). Aflatoxin exposure contributes an estimated 4.6% to 28.2% of the annual HCC cases. The highest distribution is in Africa followed by Southeast Asia and the Western Pacific nations. It is estimated that in high exposure areas of HBV, reducing exposure to non-detectable levels could reduce HCC cases by 23% (66).
Positive dietary factors
Coffee drinking in several studies including a meta-analysis has shown to reduce incidence of HCC. Coffee drinking has been associated with decreased risk of elevated enzymes and of cirrhosis. It has been shown to reduce insulin levels as well as reduce the risk of diabetes type II. The risk reduction of HCC is 40% for any coffee consumption per day verses no coffee consumption. Interestingly there appears to be a dose response relationship with a 20% reduction with 1-2 cups a day and a 75% reduction with 5 or more cups per day (67-69).
Other metabolic and genetic diseases
Hemochromatosis, alpha-1-antitrypsin disease, Wilson’s disease, tyrosinemia, citrullinemia, Type I and III glycogen storage disease, fructose intolerance, and porphyrias have an association with HCC.
Hereditary hemochromatosis (HH) is associated with increased risk of HCC (70-72). The risk has been estimated to be between 100 to 200 fold increased. This occurs predominantly in patients with cirrhosis although it may also occur in the absence of cirrhosis. Multiple studies have shown the rate of HCC in patients with HH is approximately 10% overall. HCC has also been found in other iron overload disorders. In thalassemia, HCC was reported as a late complication. However, many of those patients were also positive for hepatitis C. The majority of patients had high serum ferritin levels (>2,000).
African iron overload occurs in patients who consume noncommercial beer brewed in nongalvanized steel drums. South Africa blacks were found to have a relative risk for HCC of 10.6 in those with iron overload (transferrin saturation >60%) compared with those with normal iron stores after adjusting for alcohol, viral hepatitis and aflatoxin B1 exposure. It is inconclusive if mild to moderate iron overload associated with hepatitis C, alcohol related liver disease or the carriage of HFE mutation increases the risk of HCC in patients with cirrhosis.
Alpha-1-antitrypsin disease patients homozygous for the Z mutation (PiZZ), are at increased risk for HCC even in the absence of cirrhosis. Carriers (PiZ) may have also be at risk (73,74).
Hereditary tyrosinemia is an autosomal recess of disorder in the pediatric population which results in individuals excreting higher levels of succinylacetone into the urine and elevated tyrosine levels in the serum results in rapid development of cirrhosis and potential for HCC (75). Treatment for this disorder when started before the age of two may prevent the occurrence of HCC.
Citrullinemia another autosomal recessive of disorder presenting in young children is associated with inborn errors of the urea cycle has also been associated with HCC (76).
Tumor markers
Most HCCs when diagnosed are advanced in size/stage resulting in five-year survival rates less than 12% in the United States. When HCC is discovered early, resection in non-cirrhotic patients offers a 5-year survival rate of 70%. Transplantation within the Milan criteria (single nodule <5 cm or 3 nodules each <3 cm in diameter) offers a greater than 75% five-year survival (77). Radiofrequency ablation singularly offers a five-year survival rate in patients with early HCC for Child-Pugh A and B between 51-64% and 31-38% respectively (78-80). Unfortunately, only 30% of the tumors are discovered early enough to offer treatment with resection or transplantation. Tumor markers and surveillance in high risk populations permit earlier discovery of HCC and will improve survival. The most commonly used biomarkers at this time are Alpha-fetoprotein (AFP), AFP-L3, and Des-Gamma-Carboxy-Prothrombin (DCP). Many potentially new markers show promise, two of which are Osteopontin (OPN) and fatty acids.
Alpha-fetoprotein (AFP)
AFP, with a half-life of 5-7 days, is synthesized by embryonic liver cells, the vitelline sac, and fetal intestinal tract in the first trimester of pregnancy. Serum levels rapidly decline in the first 12 months after birth. It is the most widely investigated biomarker for HCC diagnosis. The false negative rate may be as high as 40% in patients with early HCC (<2 cm) (81). Levels may remain normal in 15-20% of patients with advanced HCC. Only 10-20% of early-stage HCC patients have abnormal AFP. Fluctuating levels occur with flare-ups of viral hepatitis without HCC. AFP cut off value of 20 ng/mL demonstrates good sensitivity but low specificity. Levels >200 ng/mL provides high specificity but markedly less sensitivity. Cut-off values for AFP in various studies ranging from 7.7 to 112 ng/mL have yielded sensitivity to be 25% to 90% and specificity between 85% and 97% (82). Increasing levels of AFP correlate with the development of HCC in cirrhotic patients. Consistently elevated levels greater than 500 ng/mL are indicative of HCC. In Alaskan hepatitis B carriers, AFP testing allowed detection of tumors in an earlier more treatable stage (83).
AFP-L3
AFP can be divided into three glycoforms. The AFP-L3 fraction expressed as a percentage of AFP reportedly is highly specific for HCC when AFP levels are greater than 20 ng/mL. AFP-L3 is correlated with a shorter tumor doubling time, an infiltrative tumor growth pattern, vascular invasion, and intrahepatic metastasis (84).
A new hypersensitive (hs) AFP-L3 has shown superior sensitivity even at AFP levels <20 ng/mL improving early detection of small tumors less than 2 cm. A cutoff value of 7% has been shown to best discriminate between benign liver disease (85). HS-AFP-L3 has been shown to be elevated one year prior to the diagnosis of HCC in 34.3% of high risk patients. Survival rate with hs-AFP-L3 >7% at one year prior to the diagnosis has been shown to be significantly lower than those patients with <7% (86).
Des-Gamma-Carboxy-Prothrombin (DCP)
DCP is produced only by malignant hepatocytes resulting from an acquired post translational defect in vitamin K dependent carboxylase system. Although independent of vitamin K deficiency, administration of vitamin K can transiently suppress DCP production (87). Levels greater than 100 ng/mL are very suggestive of HCC. DCP normalizes with successful tumor resection and has been shown to correlate with tumor activity. DCP level has the best correlation with tumors greater than 3 cm (88). DCP levels >125 mAU/mL yield the best sensitivity and specificity for differentiating HCC for chronic hepatitis and cirrhosis (89). It is more sensitive and specific than AFP for differentiating HCC from nonmalignant liver disease. High-sensitivity DCP can be used at a cutoff value of 40 mAU/mL, AFP at a cutoff of 20 ng/mL and AFP-L3 cutoff at 10% in combination gives the highest accuracy of 82.2% (sensitivity 82.1%, specificity 82.4%) (90). The combination use of AFP, DCP and AFP/AFP-L3 yields increased sensitivity in diagnosing HCC (91,92). For this reason, the Japan Society of Hepatology (JSH) recommends all three biomarkers AFP, AFP-L3 and DCP along with ultrasonography in their screening for HCC (93).
Other potential markers
Several new promising markers are in phase I/II/III studies. These include OPN, Glypican-3, Hepatocyte Growth Factor, Insulin-like growth factor, and vascular endothelial growth factor (94).
OPN with cut off values of 156 ng/mL and AFP cut-off at 20 ng/mL combined have a sensitivity of 95% and specificity of 96% for diagnosis of HCC (95). Furthermore OPN levels were elevated more than one year before diagnosis.
Screening/surveillance for HCC
Screening strategy for HCC is based on two factors, an average tumor doubling time of 3-5 months and a cost effectiveness threshold of an expected annual incidence exceeding 1.5% in cirrhosis and 0.2% in non-cirrhosis HBV patients (96). Intervention is determined to be cost effective if it does not exceed $50,000 per year of life gained. It is considered effective if it results in an increase in longevity of 100 days. Using this model, screening for most etiologies of cirrhosis is cost effective (autoimmune hepatitis cirrhosis may dip below this). The best radiologic tool that fits into this model is US examination at six months intervals. Biannual US/AFP exceeds the threshold in some studies while biannual AFP/annual contrast CT slightly exceeds the threshold by $1,750 (97). US has, in general, a sensitivity and specificity greater 60% and 90% respectively with a positive predictive value of 70% (98). The ability of US to detect a HCC nodule is dependent on their size. HCC nodules >5 cm result in detection rate of 92%. However, the detection rate decreases to 75% for 3.1-5.0 cm lesions, 20% for 2.1 to 3.0 cm lesions and 13.6% for 1-2 cm lesions (99). False positive AFP results leading to additional unnecessary tests can result in US/AFP not meeting threshold (96). US detection varies with the expertise of the person performing the examination and with the body habitus of the patient. Central obesity hampers the ability of US to detect small lesions (100). A dedicated technician may increase the detection rate of HCC. It has been shown in hepatitis B patients that US surveillance every six months improved survival (101). The five year HCC related mortality was lower in the screened group attributable to HCC detection at an earlier stage I (60% vs. 0%) allowing more patients to be treated with resection (47% vs. 8%). This is less clear in cirrhotic patients. The current AASLD guidelines recommend US every six months without AFP as the screening tool for HCC.
Who to screen (Table 2)
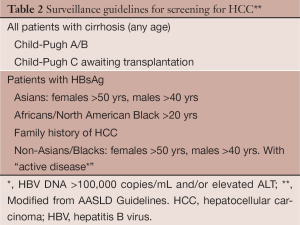
Full table
All cirrhotic patients should be screened with US for HCC. The exception to this would be the non-transplantable, decompensated (Child C) HCV cirrhosis patient whose life expectancy is too short to experience any survival effects of surveillance (102). Alcohol related cirrhosis may fall below these thresholds because of a lower incidence of HCC and the high (58%) non-HCC related mortality (103). Also, HCC screening with CT scan while awaiting liver transplantation has been shown to be associated with the greatest gain in life expectancy and is cost effective in this setting (104).
Non-cirrhotic patients who are hepatitis B carriers comprise a group of patients with individual recommendations based on cost effectiveness (incidence of HCC exceeding 0.2%). Asians are at greater risk for HCC then Caucasians (105,106). The risk of HCC in Asian male HBV carriers exceeds the threshold starting at the age of 40 yrs (107). US should be performed in Asian men over the age of 40 and Asian women over the age of 50. Black African non-cirrhotic HBV carriers have a particularly increased risk of HCC at a younger age (108). For this reason, screening should begin at the time of diagnosis or when reaching 20 years old. It is unclear if this early onset can be transferrable to non-African blacks. The guidelines are also vague in Caucasians with non-cirrhotic hepatitis B and those with a family history (FH) of HCC.
The risk of HCC in Caucasians appears to be more related to the virus inflammatory activity in non-cirrhotic patients. Surveillance should be performed in these patients with active disease as reflected by an elevated ALT and or a high viral load (>20,000 IU/mL) (109). It can be started in men at 40 years of age and in women at 50 years of age.
HBV carriers with a FH of HCC have increased risk (110). This risk increases with age (23% with HCC at 70 vs. 8.9% without a FH) and the number of family members affected (risk 5.6 times with >2 family members). Risk is independent of hepatitis however the combination of HBV or HCV serum markers plus FH increases the risk >70 times. It is unclear if there is a relationship between the age of occurrence of HCC in a family member, index case, and the age at which the risk of HCC will occur in the offspring. The age at which to start surveillance in these individuals has not been defined by the guidelines.
The diagnostic algorithms (Figure 5) once a lesion is discovered on US vary between the separate societies. Asians societies use AFP, the Americans and Europeans do not. All use US as the first screening modality. In the Asian guidelines any lesion with characteristic MR/CT enhancement is considered diagnostic for HCC while the Europeans and Americans use the size of the lesion (>1 cm). In a recent review the sensitivity and specificity for HCC diagnosis was 60% and 90% for US, 68% and 93% in the multiphasic CT, 81% and 85% in dynamic MRI. MRI showed the highest specificity. The diagnostic sensitivity depended on the size of the tumor. CT and MRI was greater than 90% sensitive for tumors 2 cm or larger, 61-65% and 80-92% tumors between 1 and 2 cm, 10% and 34-71% in tumors less than 1 cm, respectively (111). The greatest difficulty with diagnosing HCC by the various radiologic studies lies in diagnosing tumors less than 2 cm and in particular those less than 1 cm. The differential diagnosis for lesions found in a cirrhotic liver less than 2 cm is broad and includes fibrosis, regenerative nodules, cirrhotic nodules and dysplastic nodules. Lesions less than 1 cm can be confused with arterial portal shunting. Variations can exist between areas within the liver itself. Arterial vascularization in the HCC tumor is an essential key to its diagnosis on imaging studies. Arterial enhancement occurs when a dysplastic nodule becomes a frank HCC (112). MRI is better than CT at visualizing very early vascular lesions. However, well differentiated HCC may not have significant arterial enhancement in early contrast phases and may in fact have some residual hepatocyte function. This has been overcome with a new contrast agent, gadoxetic acid (Eovist in the United States, Primovist in Europe). This agent used in a delayed hepatobiliary phase (20-minute delay) results in enhancement of functioning hepatocytes that appear brighter in contrast to nonfunctioning hepatocytes i.e., HCC. In comparison studies with MRI utilizing this contrast agent to CT multi detector scans, lesions less than 2 cm were significantly more often found with MRI. Lesions 1-2 cm detection rates were 71-87% with MRI vs. 65.7-78.7% with CTMD in one comparison study and all lesion detection rates were 82-85% verses 69-71% in an additional two comparison studies (113-115).
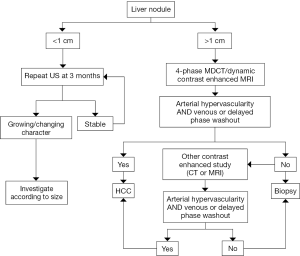
When atypical findings occur from a single imaging study the AASLD recommendations encourage the use of a second imaging modality for further assessment. If atypical findings are again found on the sequential scan biopsy is recommended. In these atypical lesions, biopsy improves specificity of imaging to 100% (116).
Biopsy of lesions less than 1 cm can be challenging. Therefore recall, repeating imaging studies, in close interval follow-up is essential. A three month interval is recommended. A longer interval, six-months, may be sufficient especially when the lesion is <5 mm noted to be subcapsular in location, ill-defined or wedge-shaped and thus more likely representing a vascular shunt. However if the lesion is noted to be round or oval, intraparenchymal or in a dominant mass a three month recall interval should be performed (117). Lesions that do not regress should be followed for two years before they are considered benign.
Screening and the guidelines: real world reality
It is important to understand that the various guidelines adopted by different societies are not dictums but suggestions. They should be followed as best as possible but sometimes reality dictates otherwise.
For surveillance to work we first need to know that the patient has cirrhosis. In a recent review in a Marketscan claims database of 729,018 patients with at least one claim for NAFLD/NASH/HCV over one quarter of the patients diagnosed with HCC had no knowledge of liver disease prior to their diagnosis (118). Amazingly, of the patients known to have liver disease, only 20.1% of patients with NASH/cirrhosis and 22.3% of those with HCV/cirrhosis were undergoing regular HCC screening.
In a retrospective analysis (HALT-C), even when patients were closely followed by expert hepatologists at academic centers one third of the patients had inconsistent HCC surveillance (119). Only 20% of the patients that developed HCC were found at a very early stage (TNM stage T1) and over one fourth of the tumors were found beyond the Milan criteria. Patients beyond stage T1 were significantly more likely to have experienced absence of screening or follow-up. Additionally, the most common reason for surveillance failure (70%) in tumors beyond the Milan criteria was absence of detection by US and AFP. This underscores the importance of the imaging study chosen and establishing an adequate patient recall program. It is also imperative to make the patient understand the importance of screening.
In our center, we conduct surveillance for HCC utilizing US alternating with MRI scan at six-month intervals in any patient whose BMI is >30. Otherwise, US at six-month intervals is performed. We utilize AFP at six-month intervals. AFP-L3 and DCP are ordered with AFP in listed and in potential transplant patients. We tell patients to use their birthday as a point of reference in time to remind him/her of the need for surveillance (birthday and six months later).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Catherine T. Frenette) for the series “Liver Cancer” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2013.12.01). The series “Liver Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma consider the population. J Clin Gastroenterol 2013;47:S2-6. [PubMed]
- El-Serag HB. Hepatocellular carcinoma. N Engl J Med 2011;365:1118-27. [PubMed]
- Jia JD, Zhuang H. A winning war against hepatitis B virus in China. Chin Med J (Engl) 2007;120:2157-8. [PubMed]
- Zanetti AR, Van Damme P, Shouval D. The global impact of vaccination against hepatitis B: a historical overview. Vaccine 2008;26:6266-73. [PubMed]
- Goh KT. Prevention and control of hepatitis B infection in Singapore. Ann Acad Med Singapore 1997;26:671-81. [PubMed]
- Kao JH, Chen DS. Recent updates in hepatitis vaccination and the prevention of hepatocellular carcinoma. Int J Cancer 2002;97:269-71. [PubMed]
- National Cancer Institute. Created on 4/2012 by Statecancerprofiles.cancer.gov: Liver and bile duct cancer death rates for United States, 2004-2008.
- Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol 2009;27:1485-91. [PubMed]
- Ramirez AG, Weiss NS, Holden AE, et al. Incidence and risk factors for hepatocellular carcinoma in Texas Latinos: implications for prevention research. PLoS One 2012;7:e35573 [PubMed]
- Yalamanchili K, Saadeh S, Lepe R, et al. The prevalence of hepatitis C virus infection in Texas: implications for future health care 2005. BUMC Proceedings;18:3-6.
- Kanwal F, Hoang T, Kramer JR, et al. Increasing prevalence of HCC and cirrhosis in patients with chronic hepatitis C virus infection. Gastroenterology 2011;140:1182-8.e1.
- El-Serag HB. Are patterns and prevalence changing? In: Williams R, Taylor-Robinson SD. eds. Clinical dilemmas in primary liver cancer. UK: Wiley-Blackwell, 2012;3-10.
- White DL, Firozi A, El-Serag HB. Epidemiology of hepatocellular carcinoma. In: Carr IB. eds. Hepatocellular carcinoma diagnosis and treatment. New York, NY: Humana Press, 2010:1-25.
- Dhir RN, Dworakowski W, Thangavel C, et al. Sexually dimorphic regulation of hepatic isoforms of human cytochrome p450 by growth hormone. J Pharmacol Exp Ther 2006;316:87-94. [PubMed]
- Justo R, Boada J, Frontera M, et al. Gender dimorphism in rat liver mitochondrial oxidative metabolism and biogenesis. Am J Physiol Cell Physiol 2005;289:C372-8. [PubMed]
- Rogers AB, Theve JT, Feng Y, et al. Hepatocellular Carcinoma associated with liver–gender disruption in male mice. Cancer Res 2007;67:11536-46. [PubMed]
- Yuan JM, Ross RK, Stanczyk FZ, et al. A cohort study of serum testosterone and hepatocellular carcinoma end Shang-hai, China. Int J Cancer 1995;63:491-3. [PubMed]
- White DL, Tavakoli-Tabasi S, Kuzniarek J, et al. Higher serum testosterone is associated with increased risk of advanced hepatitis C related liver disease in males. Hepatology 2012;55:759-68. [PubMed]
- Wu MH, Ma WL, Hsu CL, et al. Androgen receptor promotes hepatitis B virus-induced hepatocarcinogenesis through modulation of hepatitis B virus RNA transcription. Sci Transl Med 2010;2:32ra35 [PubMed]
- Naugler WE, Sakurai T, Kim S, et al. Gender disparity in liver cancer due to sex differences and MyD88–dependent IL-6 production. Science 2007;317:121-4. [PubMed]
- Tavani A, Negri E, Parazzini F, et al. Female hormone utilization and risk of hepatocellular carcinoma. Br J Cancer 1993;67:635-7. [PubMed]
- Fiel MI, Min A, Gerber MA, et al. Hepatocellular carcinoma in long-term oral contraceptive use. Liver 1996;16:372-6. [PubMed]
- Neuberger J, Forman D, Doll R, et al. Oral contraceptives and hepatocellular carcinoma. Br Med J (Clin Res Ed) 1986;292:1355-7. [PubMed]
- Parkin DM. The Global health burden of infection–associated cancers in the year 2002. Int J Cancer 2006;118:3030-44. [PubMed]
- Shi J, Zhu L, Liu S, et al. K Mehta analysis of case–control studies on the combined effect of hepatitis B and C virus infections in causing hepatocellular carcinoma in China. Br J Cancer 2005;92:607-12. [PubMed]
- Seeger C, Mason WS. Hepatitis B virus biology. Microbiol Mol Biol Rev 2000;64:51-68. [PubMed]
- Chen CJ, Yang HI, Su J, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 2006;295:65-73. [PubMed]
- Yu MW, Yeh SH, Chen PJ, et al. Hepatitis B virus genotype and DNA level and hepatocellular carcinoma: a prospective study in men. J Natl Cancer Inst 2005;97:265-72. [PubMed]
- Chen CJ, Yang HI, Iloeje UH, et al. Hepatitis B virus DNA levels and outcomes in chronic hepatitis B. Hepatology 2009;49:S72-84. [PubMed]
- Chen G, Lin W, Shen F, et al. Past HBV viral load as predictor of mortality and morbidity from HCC in chronic liver disease in a prospective study. Am J Gastroenterol 2006;101:1797-803. [PubMed]
- Chen CJ, Liang KY, Chang AS, et al. Effects of hepatitis B virus, alcohol drinking, cigarette smoking and familial tendency on hepatocellular carcinoma. Hepatology 1991;13:398-406. [PubMed]
- Ohnishi K, Lida S, Iwama S, et al. The effect of chronic habitual alcohol intake on the development of the liver cirrhosis and hepatocellular carcinoma: Relation to hepatitis B surface antigen carriage. Cancer 1982;49:672-7. [PubMed]
- Pereira FE, Goncalves CS, Zago MP. The effect of ethanol intake on the development of hepatocellular carcinoma and HBsAg carriers. Arq Gastroenterol 1994;31:42-6. [PubMed]
- Hosaka T, Suzuki F, Kobayashi M, et al. Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology 2013;58:98-107. [PubMed]
- But DYK, Lai CL, Yuen MF. The natural history of hepatitis related hepatocellular carcinoma. World J Gastroenterol 2008;14:1652-6. [PubMed]
- Lok AS, Seeff LB, Morgan TR, et al. Incidence of hepatocellular carcinoma and associated risk factors and hepatitis C related advanced liver disease. Gastroenterology 2009;136:138-48. [PubMed]
- Shepard CW, Finelli L, Alter MJ, et al. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis 2005;5:558-67. [PubMed]
- Armstrong GL, Wasley A, Simard EP, et al. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med 2006;144:705-14. [PubMed]
- Tanaka Y, Kurbanov F, Mano S, et al. Molecular tracing of the Global hepatitis C virus epidemic predicted regional patterns of hepatocellular carcinoma mortality. Gastroenterology 2006;130:703-14. [PubMed]
- Armstrong GL, Alter MJ, McQuillan GM, et al. The past incidence of hepatitis C virus infection: Implications for future burden of chronic liver disease in the United States. Hepatology 2000;31:777-82. [PubMed]
- Puoti M, Bruno R, Soriano V, et al. Hepatocellular carcinoma in HIV–infected patient’s colon epidemiological features, clinical presentation and outcome. AIDS 2004;18:2285-93. [PubMed]
- Singal AK, Anand BS. Mechanisms of synergy between alcohol and hepatitis C virus. J Clin Gastroenterol 2007;41:761-2. [PubMed]
- Morgan RL, Baak B, Smith BD, et al. Eradication of hepatitis C virus infection in the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Int Med 2013;158:329-37. [PubMed]
- Starley BQ, Calagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology 2010;51:1820-32. [PubMed]
- Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospective studied cohort of U.S. adults. N Engl J Med 2003;348:1625-38. [PubMed]
- Larsson SC, Wolk A. Overweight, obesity and risk of liver cancer: a meta-analysis of cohort studies. Br J Cancer 2007;97:1005-8. [PubMed]
- Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD single topic conference. Hepatology 2003;37:1202-19. [PubMed]
- Williams CD, Stengel J, Asike ML, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology 2011;140:124-31. [PubMed]
- Davila JA, Morgan RO, Shaib Y, et al. Diabetes increases the risk of hepatocellular carcinoma in the United States: a population based case control study. Gut 2005;54:533-9. [PubMed]
- El-Serag HB, Tran T, Everhart JE, et al. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology 2004;126:460-8. [PubMed]
- Sanyal A, Poklepovic A, Moyneur E, et al. Population based risk factors and resource utilization for HCC: U.S. perspective. Curr Med Res Opin 2010;26:2183-91. [PubMed]
- Ertle J, Dechene A, Sowa JP. Nonalcoholic fatty liver disease progresses to hepatocellular carcinoma in the absence of apparent cirrhosis. Int J Cancer 2011;128:2436-43. [PubMed]
- Gyorgy B, Brunt EM, Caldwell SH. Hepatocellular carcinoma in nonalcoholic fatty liver disease: an emerging menace. J Hepatology 2012;56:1384-91.
- Caldwell SH, Lee VD, Kleiner DE, et al. NASH and cryptogenic cirrhosis: a histological analysis. Ann Hepatol 2009;8:346-52. [PubMed]
- Caldwell SH, Oelsner DH, Iezzone JC, et al. Cryptogenic cirrhosis: clinical characterization and risk factors for underlying disease. Hepatology 1999;29:664-9. [PubMed]
- Poonawala A, Nair SP, Thuluvath PJ, et al. Prevalence of obesity and diabetes in patients with cryptogenic cirrhosis: a case control study. Hepatology 2000;32:689-92. [PubMed]
- Gao C, Fang L, Zhao HC, et al. Potential role of diabetes mellitus in the progression of cirrhosis to hepatocellular carcinoma: a cross-sectional case control study from Chinese patients with HBV infection. Hepatobiliary Pancreat Dis Int 2013;12:385-93. [PubMed]
- Wang C, Wang X, Gong G, et al. Increased risk of hepatocellular carcinoma in patients with diabetes mellitus: a systemic review and meta-analysis of cohort studies. Int J Cancer 2012;130:1639-48. [PubMed]
- Jee SH, Ohrr H, Sull JW, et al. Fasting serum glucose level and cancer risk in Korean men and women. JAMA 2005;293:194-202. [PubMed]
- Morgan TR, Mandayam S, Jamal MM. Alcohol and hepatocellular carcinoma. Gastroenterology 2004;127:S87-96. [PubMed]
- Jewell J, Sheron N. Trends in European liver death rates: Implications for alcohol policy. Clin Med 2010;10:259-63. [PubMed]
- Donato F, Tagger A, Gelatti U, et al. Alcohol and hepatocellular carcinoma: the effect of lifetime intake and hepatitis virus infections in men and women. Am J Epidemiol 2002;155:323-31. [PubMed]
- Hassan MM, Hwang LY, Hatten CJ, et al. Risk factors for hepatocellular carcinoma: synergy some of alcohol with viral hepatitis and diabetes mellitus. Hepatology 2002;36:1206-13. [PubMed]
- Wu HC, Santella R. The role of aflatoxins in hepatocellular carcinoma. Hepat Mon 2012;12:e7238 [PubMed]
- Lui Y, Wu F. Global burden of aflatoxin-induced hepatocellular carcinoma: a risk assessment. Environ Health Pers 2010;118:818-24.
- Liu Y, Chang CC, Marsh GM, et al. Population attributable risk of aflatoxin related liver cancer: systemic review and meta-analysis. Eur J Cancer 2012;48:2125-36. [PubMed]
- Bravi F, Bosetti C, Tavani A, et al. Coffee drinking and hepatocellular carcinoma risk: a meta-analysis. Hepatology 2007;46:430-5. [PubMed]
- Bravi F, Bosetti C, Tavani A, et al. Coffee reduces risk for hepatocellular carcinoma: an updated meta-analysis. Clin Gastroenterol Hepatol 2013;11:1413-21. [PubMed]
- Inoue M, Yoshimi I, Sobue T, et al. Influence of coffee drinking on subsequent risk of hepatocellular carcinoma: a prospective study in Japan. J Natl Cancer Inst 2005;97:293-300. [PubMed]
- Kowdley KV. Iron, hemochromatosis, and hepatocellular carcinoma. Gastroenterology 2004;127:S79-86. [PubMed]
- Ko C, Siddaiah N, Berger J, et al. Prevalence of hepatic iron overload and association with hepatocellular cancer and end-stage liver disease: results from a national hemochromatosis transplant registry. Liver Int 2007;27:1394-401. [PubMed]
- Deugnier Y, Turin B. Iron and hepatocellular carcinoma. J Gastroenterol Hepatol 2001;16:491-4. [PubMed]
- Fairbanks KD, Tavill AS. Liver disease and alpha-1 antitrypsin deficiency: a review. Am J Gastroenterol 2008;103:2136-41. [PubMed]
- Rudnick DA, Perlmutter DH. Alpha-1 antitrypsin deficiency: a new paradigm for hepatocellular carcinoma in genetic liver disease. Hepatology 2005;42:514-21. [PubMed]
- Hassan M. EL-Shabrawi, Kamal NM. Current management options for tyrosinemia. ODRR 2013;3:1-9.
- Ito T, Shiraki K, Sekoguchi K, et al. Hepatocellular carcinoma associated with adult-type citrullinemia. Dig Dis Sci 2000;45:2203-6. [PubMed]
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693-9. [PubMed]
- Lencioni R, Cioni D, Crocetti L, et al. Early-stage hepatocellular carcinoma in cirrhosis: long-term results of percutaneous image-guided radiofrequency ablation. Radiology 2005;234:961-7. [PubMed]
- Tateishi R, Shiina S, Teratani T, et al. Percutaneous radiofrequency ablation for hepatocellular carcinoma. Cancer 2005;103:1201-9. [PubMed]
- Choi D, Lim HK, Rhim H, et al. Percutaneous radiofrequency ablation for early-stage hepatocellular carcinoma as a first line treatment: Long-term results and prognostic factors in a large single institution series. Eur Radiol 2007;17:684-92. [PubMed]
- Wang L, Yao M, Dong Z, et al. Circulating specific biomarkers and diagnosis of hepatocellular carcinoma and its metastasis monitoring. Tumour Biol 2013; [Epub ahead of print]. [PubMed]
- Witjes CD, van Aalten SM, Steyerberg EW, et al. Recently introduced biomarkers for screening of hepatocellular carcinoma: a systemic review and meta-analysis. Hepatol Int 2013;7:59-64. [PubMed]
- McMahon BJ, Bulkow L, Harpster A, et al. Screening for hepatocellular carcinoma in Alaskan natives infected with chronic hepatitis B: a 16 year population-based study. Hepatology 2000;32:842-6. [PubMed]
- Khien VV, Mao HV, Chinh TT, et al. Clinical evaluation of lentil lectin-reactive alpha fetoproteins–L3 in histology-proven hepatocellular carcinoma. Int J Biol Markers 2001;16:105-11. [PubMed]
- Tamura Y, Igarashi M, Kawai H, et al. Clinical advantage of high sensitivity on-chip immunoassay for fucosylated fraction of alpha-fetoprotein in patients with hepatocellular carcinoma. Dig Dis Sci 2010;55:3576-83. [PubMed]
- Kumada T, Toyoda H, Tada T, et al. High-sensitivity Lens culinaris agglutinin-reactive alpha-fetoprotein assay predicts early detection of hepatocellular carcinoma. J Gastroenterol 2013; [Epub ahead of print]. [PubMed]
- Behne T, Copur MS. Biomarkers for hepatocellular carcinoma. Int J Hepatology 2012;2012:1-7.
- Weitz IC, Liebman HA. Des-g-carboxy (abnormal) prothrombin and hepatocellular carcinoma: a critical review. Hepatology 1993;18:990-7. [PubMed]
- Marrero JA, Su GL, Wei W, et al. Des –gamma- carboxy prothrombin can differentiate hepatocellular carcinoma from nonmalignant chronic liver disease in American patients. Hepatology 2003;37:1114-21. [PubMed]
- Singhal A, Jayaraman M, Dhanaserkaran DN, et al. Molecular and serum markers in hepatocellular carcinoma: predictive tools for prognosis and recurrence. Crit Rev Oncol Hematol 2012;82:116-40. [PubMed]
- Ertle JM, Heider D, Wichert M, et al. A combination of alpha-fetoprotein and des-gamma-carboxy prothrombin is superior in detection of hepatocellular carcinoma. Digestion 2013;87:121-31. [PubMed]
- Choi JY, Jung SW, Kim HY, et al. Diagnostic value of AFP-L3 and PIVKA-II in hepatocellular carcinoma according to total-AFP. World J Gastroenterol 2013;19:339-46. [PubMed]
- Makuuchi M, Kokudo N, Arii S, et al. Development of evidenced- based clinical guidelines for the diagnosis and treatment or hepatocellular carcinoma in Japan. Hepatol Res 2008;38:37-51. [PubMed]
- Masuzaki R, Karp SJ, Omata M. New serum markers of hepatocellular carcinoma. Semin Oncol 2012;39:434-9. [PubMed]
- Shang S, Plymonth A, Ge S, et al. Identification of Osteopontin as a novel marker for early hepatocellular carcinoma. Hepatology 2012;55:483-90. [PubMed]
- Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020-2. [PubMed]
- Lin OS, Keeffe EB, Sanders GD, et al. Cost-effectiveness or screening for hepatocellular carcinoma in patients with cirrhosis due to chronic hepatitis C. Aliment Pharmacol Ther 2004;19:1159-72. [PubMed]
- Daniele B, Bencivenga A, Megna AS, et al. Alpha-fetoprotein and ultrasonography screening for hepatocellular carcinoma. Gastroenterology 2004;127:S108-112. [PubMed]
- Bennett GL, Krinsky GA, Abitbol RJ, et al. Sonographic detection of hepatocellular carcinoma and dysplastic nodules in cirrhosis: correlation of pretransplantation sonography and liver explant pathology in 200 patients. AJR Am J Roentgenol 2002;179:75-80. [PubMed]
- Brahee DD, Ogedegbe C, Hassler C, et al. Body mass index and abdominal image quality. J Diagnos Med Sonography 2013;29:66-72.
- Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol 2004;130:417-22. [PubMed]
- Trevisani F, Santi V, Gramenzi A, et al. Surveillance for early diagnosis of hepatocellular carcinoma: is it effective in intermediate/advanced cirrhosis? Am J Gastroenterol 2007;102:2448-57. [PubMed]
- Jepsen P, Ott P, Anderson PK, et al. Clinical coarse of alcoholic liver cirrhosis: a Danish population-based cohort study. Hepatology 2010;51:1675-82. [PubMed]
- Saab S, Ly D, Nieto J, et al. Hepatocellular carcinoma screening in patients waiting for liver transplantation: a decision analytical model. Liver Transpl 2003;9:672-81. [PubMed]
- Sakuma K, Saitoh N, Kasai M, et al. Relative risks of death due to liver disease among Japanese male adults having various statuses for hepatitis B s and e antigen/antibody in serum: a prospective study. Hepatology 1988;8:1642-6. [PubMed]
- Kew MC. Epidemiology of chronic hepatitis B virus infection, hepatocellular carcinoma, and hepatitis B virus-induced hepatocellular carcinoma. Pathol Biol (Paris) 2010;58:273-7. [PubMed]
- Beasley R. Hepatitis B virus as the etiologic agent in hepatocellular carcinoma. Hepatology 1982;2:21S-26S.
- Kew MC, Macerollo P. Effect of age on the etiologic role of the hepatitis B virus in hepatocellular carcinoma in blacks. Gastroenterology 1988;94:439-42. [PubMed]
- Lok AS, Heathcote EJ, Hoofnagle JH. Management of hepatitis B: 2000 summary of a workshop. Gastroenterology 2001;120:1828-53. [PubMed]
- Yu MW, Chang HC, Liaw YF, et al. Familial risk of hepatocellular carcinoma among chronic hepatitis B carriers and their relatives. J Natl Cancer Inst 2000;92:1159-64. [PubMed]
- Colli A, Fraquelli M, Casazza G, et al. Accuracy of Ultrasonography, spiral CT, magnetic resonance, and alpha-fetoprotein in diagnosing hepatocellular carcinoma: a systemic review. Am J Gastroenterol 2006;101:513-23. [PubMed]
- Mion F, Grozel L, Boillot O, et al. Adult cirrhotic liver explants: precancerous lesions and undetected small hepatocellular carcinomas. Gastroenterology 1996;111:1587-92. [PubMed]
- Ichikawa T, Saito K, Yoshoika N, et al. Detection and characterization of focal liver lesions: a Japanese phase III, multicenter comparison between gadoxetic acid disodium-enhanced magnetic resonance imaging and contrast-enhanced computed tomography predominately in patients with hepatocellular carcinoma and chronic liver disease. Invest Radiol 2010;45:133-41. [PubMed]
- Di Martino M, Marin D, Guerrisi A, et al. Intra individual comparison of gadoxetate disodium-enhanced MR imaging and 64-section multi detector CT in the detection of hepatocellular carcinoma in patients with cirrhosis. Radiology 2010;256:806-16. [PubMed]
- Hammerstingl R, Huppertz A, Breuer JEuropean EOB-study group, et al. Diagnostic efficacy of gadoxetic acid (Primovist)-enhanced MRI and spiral CT for a therapeutic strategy: comparison with intraoperative and histopathologic findings in focal liver lesions. Eur Radiol 2008;18:457-67. [PubMed]
- Leoni S, Piscaglia F, Golfieri R, et al. The impact of vascular and nonvascular findings on the noninvasive diagnosis small hepatocellular carcinoma based on EASL and AASLD criteria. Am J Gastroenterol 2010;105:599-609. [PubMed]
- Willatt JM, Hussain HK, Adusumilli S, et al. MR imaging of hepatocellular carcinoma in the cirrhotic liver: challenges and controversies. Radiology 2008;247:311-30. [PubMed]
- Sanyal A, Poklepovic A, Moyneur E, et al. Population- based risk factors and resource utilization for HCC: US perspective. Curr Med Res Opin 2010;26:2183-91. [PubMed]
- Singal AG, Nehra M, Adams-Huet B, et al. Detection of hepatocellular carcinoma at advanced stages among patients in the HALT-C trial: where did surveillance fail? Am J Gastroenterol 2013;108:425-32. [PubMed]


