
Vitamin D receptor and cyclooxygenase-2 expression in uterine leiomyoma tissues and their correlation
Introduction
Uterine leiomyomas are the most common gynecological benign tumors in females, clinically affecting more than 25% of reproductive age females and causing significant morbidity (1,2). Although some leiomyomas don’t cause any symptoms, symptomatic leiomyomas are usually associated with specific symptoms such as anemia, abnormal uterine bleeding, abdominal distention, pelvic pain and frequent micturition. Uterine leiomyomas are the most common diseases for hysterectomy in the United States and they cost at least 6 billion dollars annually (3). Despite the fact that uterine leiomyomas are very common and cause significant medical and economic burdens, the etiology is still partially understood. The occurrence and development of uterine fibroids may be relevant to certain risk factors such as estrogen, progestogen, growth factors and ethnicity, but the exact causes are not yet well known (4,5). Recently, some studies indicated that lower serum vitamin D levels are inversely relevant to leiomyoma burden in different ethnic groups and vitamin D inadequacy may be associated with the occurrence of uterine leiomyomas (6,7). Inflammation can be considered as the 7th hallmark of cancer (8). The causality between the inflammation and tumor proliferation may be explained that the injury caused by the infection causes cellular proliferation and the extracellular matrix growth due to pro-inflammatory and growth factors. Furthermore, it decreases cellular apoptosis and abnormal tissue repair. And chronic inflammation may be a risk for the development of uterine leiomyomas (9). Cyclooxygenase-2 (COX-2) plays a key role in inflammatory processes and in control of cell growth, and it has also been associated with tumorigenesis. Vitamin D can inhibit the inflammatory process by vitamin D receptor (VDR). So far, there are few studies about VDR and COX-2 concomitant expression in uterine leiomyomas. In this study, we found that VDR expression decreased and COX-2 expression increased in uterine leiomyomas, and there was a certain correlation between VDR and COX-2, which may play a role in the etiopathogenesis of uterine leiomyomas.
Methods
Patients and tissues
The study was approved by Institutional Review Board of China-Japan Friendship Hospital (No. 2016-GZR-16), and it was agreed by each patient. Written informed consent was obtained from every patient before the study began. Specimens of benign leiomyomas and adjacent matched normal myometrium tissues were obtained from 20 premenopausal women undergoing hysterectomy or myomectomy. Normal myometrium tissues were harvested at least 1cm far from the leiomyomas. The age of patients ranged from 31 to 53 years old. None of them had received any hormone, vitamin D or vitamin D analog therapy for at least 3 months before the surgery. The size of uterine leiomyomas was determined by transvaginal ultrasound or scale during the surgery. Volume of all leiomyoma lesions were determined according to the formula (a × b × c × 0.523) (6), where a is length, b is width, and c is height. Tissue specimens were taken from the nucleus of intramural leiomyomas and adjacent normal myometrium tissues. Tissue specimens were taken into ice-cold box and immediately sent to the laboratory.
Reagents and antibodies
Trizol reagent was ordered from Invitrogen. RT-PCR kit was purchased from Takara. VDR rabbit monoclonal antibody (anti-VDR), COX-2 rabbit monoclonal antibody (anti-COX-2) and GAPDH rabbit monoclonal antibody was ordered from Cell Signaling Technology. Horseradish peroxidase-labeled goat anti-rabbit IgG, RIPA buffer and SDS-PAGE sample loading buffer was purchased from Beyotime Biotechnology.
RNA extraction and quantitative real-time PCR
Total RNA was extracted from the tissues using Trizol reagent according to manufacturer’s protocol. The concentration of total RNA was examined using a NanoDrop 2000 system (Gene Company Limited, China). Five micrograms of total RNA were reverse transcribed in a 40-µL volume using an quantitative RT-PCR kit. After incubation at 42 °C for 60 min, the reverse transcription reaction was finished by heating at 75 °C for 5 min. The synthesized cDNA was amplified by quantitative real-time PCR (qPCR). qPCR was conducted using Stratagene Mx3000P (Agilent Technologies, USA) according to the manufacturer’s instructions. Primer sequences of genes were as follows: VDR primer, forward: 5'-GTGGACATCGG CATGATGAAG-3', reverse: 5'-GGTCGTAGGTCTTATGGTGGG-3'; COX-2 primer, forward: 5'-TCAAGTCCCTGAGCATCTACGGTT-3', reverse: 5'-CTGTTG TGTTCCCGCAGCCAGATT-3'; GAPDH primer, forward: 5'-GGACCTGACCTGC CGTCTAG-3', reverse: 5'-TAGCCCAGGATGCCCTTGAG-3’. qPCR was performed with the following cycling conditions: 95 °C for 2 min, 40 cycles of 95 °C for 15 s and 60 °C for 60 s. GAPDH expression was used as internal control to standardize the VDR and COX-2 results. The experiment was repeated 3 times with two replicates for each specimen.
Western blotting analysis
Total protein was extracted from the tissues followed the manufacturer’s instructions. And protein concentrations were examined using Bio-Rad protein assay reagents according to the manufacturer’s instructions. Samples were resolved by 10% SDS-PAGE. Protein samples were transferred onto polyvinylidene fluoride membranes and the membranes were immersed with specific primary antibodies respectively (anti-VDR, 1:1,000; anti-COX-2, 1:1,000). Specific protein bands were observed after exposure to enhanced chemiluminescence reagent and by E-Gel Imager. The band intensity of each protein was evaluated by image analysis software and normalized against corresponding GAPDH. The experiment was repeated three times for each specimen.
Statistical analysis
Data were expressed as mean ± standard deviation. Differences in the expression levels of VDR and COX-2 in uterine leiomyoma tissues and healthy uterine smooth muscle tissues were performed by paired t-test using SPSS 20.0 software. The correlation between VDR expression and COX-2 expression was performed by Pearson correlation test. P value <0.05 was considered statistically significant at a 95% confidence level.
Results
VDR expression in uterine leiomyoma tissues
The expression of VDR mRNA in uterine leiomyoma tissues and their paired adjacent healthy myometrium tissues were determined by qPCR. The expression of VDR mRNA in 15 of 20 uterine leiomyoma tissues decreased compared with the healthy myometrium tissues. Relative expression of VDR mRNA in 20 uterine leiomyoma tissues were significantly lower than that in healthy myometrium tissues (P<0.05) (Figure 1). The other hand, VDR proteins were also determined by Western blotting. The result showed the same trend of VDR expression as mRNA level. The protein level of VDR in leiomyoma tissues were significantly lower than that in normal myometrium tissues (Figure 2).
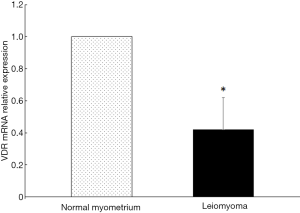
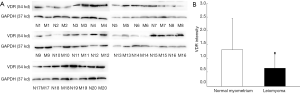
COX-2 expression in uterine leiomyomas tissues
The expression of COX-2 mRNA in uterine leiomyoma tissues and their paired adjacent healthy uterine smooth muscle tissues were determined by qPCR. The expression of COX-2 mRNA in 13 of 20 uterine leiomyoma tissues increased compared with the healthy uterine smooth muscle tissues. Relative expression of COX-2 mRNA in 20 uterine leiomyoma tissues were significantly higher than that in healthy uterine smooth muscle tissues (P<0.05) (Figure 3). The Western blotting result also showed COX-2 protein expressions in leiomyoma tissues were significantly higher than that in healthy uterine smooth muscle tissues (Figure 4).
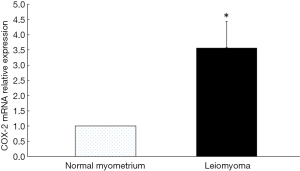
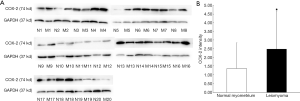
The correlation between VDR expression and COX-2 expression
We supposed that there was some correlation between VDR expression and COX-2 expression. Among the 20 cases, there was not significant correlation between VDR and COX-2 expression (r=−0.209, P>0.05 for transcriptional level; r=−0.165, P>0.05 for protein level). However, among the 11 cases with VDR expression decreased and COX-2 expression increased in uterine leiomyoma tissues, there was negative correlation between VDR and COX-2 expression (r=−0.628, P<0.05 for transcriptional level; r=−0.612, P<0.05 for protein level).
The correlation between VDR expression, COX-2 expression and uterine leiomyoma size
The diameter of the leiomyomas was from 3.5 to 9.2 cm, and the volume ranged from 16.03 to 205.99 cm3. There was not significant correlation between VDR expression and uterine leiomyoma size, as well as between COX-2 expression and uterine leiomyoma size (r=−0.008, P>0.05; r=0.150, P>0.05, respectively).
Discussion
Vitamin D mainly consists of two highly lipophilic substances: cholecalciferol (vitamin D3) and ergocalciferol (vitamin D2), which is barely active. It needs further activation steps to become the biologically active 1α,25-dihydroxyvitamin D3 [1,25(OH)2D3]. VDR is the natural ligand of 1,25(OH)2D3, which is a transcriptional factor of the nuclear receptor superfamily (10). Vitamin D plays a physiological role by liganded VDR binding to vitamin D response elements in the promoter and regulatory region of vitamin D target genes (11). The main function of serum 1,25(OH)2D3 is regulation of bone mineralization and calcium/phosphate homeostasis (12). Autocrine and paracrine actions of the locally synthesized 1,25(OH)2D3 include improving of the innate immune system, lowering autoimmune disease, reducing risk of heart attack, and exerting antitumorigenic activities (13). What’s more, many studies demonstrate that 1,25(OH)2D3 is able to delay tumor formation and prevent spread of metastases by altering cellular energy metabolism, prohibiting proliferation, inducing differentiation, activating apoptosis and inhibiting angiogenesis of the tumor (14,15). However, human clinical data are much less conclusive in showing cancer preventive effects of vitamin D (16). Due to the influence on tumors, it interests the researchers to investigate the role of vitamin D in the pathogenesis of uterine leiomyomas. Some studies demonstrated that the serum vitamin D of the persons with uterine leiomyomas were significantly lower than that of the persons without leiomyomas and vitamin D may play a role in the occurrence and development of uterine leiomyomas (17,18). Additionally, in vitro and animal studies indicated that vitamin D could inhibit uterine leiomyoma cells growth and leiomyomas shrinked by vitamin D treatment (19,20). So far, the mechanism of vitamin D impacting on uterine leiomyomas is unknown. Vitamin D performs physiological role by VDR, but VDR expression in uterine leiomyomas are not verified. Only a few researches found that most uterine fibroid tissues expressed low levels of VDR compared to adjacent normal myometrium, and there was inverse correlation between higher levels of estrogen receptor, progesterone receptor and lower levels of VDR (19,21). We also found that the expression of VDR in uterine leiomyomas was significantly lower compared to adjacent normal myometrium. Although one study demonstrated that serum levels of vitamin D3 are inversely related to leiomyoma sizes (6), the correlation between VDR and leiomyoma sizes is unknown. In this study the result indicated that there was not significant correlation between VDR and leiomyoma sizes.
Prostaglandin signaling plays an important role in inflammation-associated tumor formation (22). Prostaglandin E2 is a lipid mediator that is synthesized by COX-2 in all cell types. COX-2 is inducible by inflammatory stimuli, including cytokines, growth factors, and tumor promoters, and is upregulated in a variety of malignancies and favors the growth of malignant cells by stimulating proliferation and angiogenesis (23-25). The expression of COX-2 and its role in uterine leiomyomas is not conclusive. There are a few reports that COX-2 expression in uterine leiomyomas increased significantly compared to normal myometrium tissues (26,27), while other study indicated that COX-2 expression in smooth muscle tumors is not a prominent mark (28). In addition, celecoxib could inhibit the leiomyoma cell growth by blocking the COX-2 inflammatory pathway, suggesting that COX-2 plays an important role in the pathogenesis of uterine fibroids (27,29). In this study we found that the expression of COX-2 in 13 of 20 uterine leiomyoma tissues was higher than in the healthy uterine smooth muscle tissues. It was in accordance with the previous studies.
Some researches demonstrated that there was a negative correlation between VDR and COX-2 in cancerous tissues (30,31). And in vitro study showed that 1,25(OH)2D3 significantly inhibited inflammatory responses, such as IL-8 expression and prostaglandin activity (32). In mice, calcitriol inhibited COX-2 expression and reduced subsequent prostaglandin synthesis in macrophages (33). However, to date there is no study examined the correlation of VDR with COX-2 in human uterine leiomyomas. We postulated that there was an inverse relevance between VDR and COX-2 in uterine leiomyomas. And vitamin D may play a role in leiomyomas by the COX-2 signaling pathway. Unexpectedly, we found that there was not significant correlation between VDR and COX-2 expression in leiomyomas among the total specimens. However, among the 11 cases with VDR expression decreased and COX-2 expression increased in uterine leiomyoma tissues, there was an inverse relevance between VDR and COX-2 expression. As far as we were concerned, the small quantity of the specimens led to the discrepancy. The inverse correlation of VDR with COX-2 may be confirmed by another big population study in future.
In conclusion, in this study we found that VDR expression decreased and COX-2 expression increased in uterine leiomyomas compared with normal myometrium tissues. Decreased VDR expression and high COX-2 expression may play a role in the pathogenesis of uterine leiomyomas. There may be an inverse correlation between VDR and COX-2 in leiomyomas.
Acknowledgments
We thank all the physicians and nurses who participated in this study.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.01.19). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of China-Japan Friendship Hospital (No. 2016-GZR-16). Written informed consent was obtained from every patient before the study began.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Manta L, Suciu N, Toader O, et al. The etiopathogenesis of uterine fibromatosis. J Med Life 2016;9:39-45. [PubMed]
- Khan AT, Shehmar M, Gupta JK. Uterine fibroids: current perspectives. Int J Womens Health 2014;6:95-114. [Crossref] [PubMed]
- Cardozo ER, Clark AD, Banks NK, et al. The estimated annual cost of uterine leiomyomata in the United States. Am J Obstet Gynecol 2012;206:211.e1-9. [Crossref] [PubMed]
- Borahay MA, Al-Hendy A, Kilic GS, et al. Signaling Pathways in Leiomyoma: Understanding Pathobiology and Implications for Therapy. Mol Med 2015;21:242-56. [Crossref] [PubMed]
- Catherino WH, Eltoukhi HM, Al-Hendy A. Racial and ethnic differences in the pathogenesis and clinical manifestations of uterine leiomyoma. Semin Reprod Med 2013;31:370-9. [Crossref] [PubMed]
- Sabry M, Halder SK, Allah AS, et al. Serum vitamin D3 level inversely correlates with uterine fibroid volume in different ethnic groups: a cross-sectional observational study. Int J Womens Health 2013;5:93-100. [PubMed]
- Paffoni A, Somigliana E, Vigano P, et al. Vitamin D status in women with uterine leiomyomas. J Clin Endocrinol Metab 2013;98:E1374-8. [Crossref] [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [Crossref] [PubMed]
- Rogers R, Norian J, Malik M, et al. Mechanical homeostasis is altered in uterine leiomyoma. Am J Obstet Gynecol 2008;198:474.e1-11. [Crossref] [PubMed]
- Carlberg C, Molnar F. Current status of vitamin D signaling and its therapeutic applications. Curr Top Med Chem 2012;12:528-47. [Crossref] [PubMed]
- Ramagopalan SV, Heger A, Berlanga AJ, et al. A ChIP-seq defined genome-wide map of vitamin D receptor binding: Associations with disease and evolution. Genome Res 2010;20:1352-60. [Crossref] [PubMed]
- Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266-81. [Crossref] [PubMed]
- Haussler MR, Whitfield GK, Kaneko I, et al. Molecular mechanisms of vitamin D action. Calcif Tissue Int 2013;92:77-98. [Crossref] [PubMed]
- Feldman D, Krishnan AV, Swami S, et al. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer 2014;14:342-57. [Crossref] [PubMed]
- Abdelbaset-Ismail A, Pedziwiatr D, Suszyńska E, et al. Vitamin D3 stimulates embryonic stem cells but inhibits migration and growth of ovarian cancer and teratocarcinoma cell lines. J Ovarian Res 2016;9:26. [Crossref] [PubMed]
- Gröschel C, Tennakoon S, Kállay E. Cytochrome P450 Vitamin D Hydroxylases in Inflammation and Cancer. Adv Pharmacol 2015;74:413-58. [Crossref] [PubMed]
- Ciebiera M, Włodarczyk M, Słabuszewska-Jóźwiak A, et al. Influence of vitamin D and transforming growth factor β3 serum concentrations, obesity, and family history on the risk for uterine fibroids. Fertil Steril 2016;106:1787-92. [Crossref] [PubMed]
- Wise LA, Ruiz-Narváez EA, Haddad SA, et al. Polymorphisms in vitamin D-related genes and risk of uterine leiomyomata. Fertil Steril 2014;102:503-10.e1. [Crossref] [PubMed]
- Halder SK, Osteen KG, Al-Hendy A. Vitamin D3 inhibits expression and activities of matrix metalloproteinase-2 and -9 in human uterine fibroid cells. Hum Reprod 2013;28:2407-16. [Crossref] [PubMed]
- Halder SK, Sharan C, Al-Hendy A. 1,25-dihydroxyvitamin D3 treatment shrinks uterine leiomyoma tumors in the Eker rat model. Biol Reprod 2012;86:116. [Crossref] [PubMed]
- Al-Hendy A, Diamond MP, El-Sohemy A, et al. 1,25-Dihydroxyvitamin D3 Regulates Expression of Sex Steroid Receptors in Human Uterine Fibroid Cells. J Clin Endocrinol Metab 2015;100:E572-82. [Crossref] [PubMed]
- Nakanishi M, Rosenberg DW. Multifaceted roles of PGE2 in inflammation and cancer. Semin Immunopathol 2013;35:123-37. [Crossref] [PubMed]
- Basu S, Combe K, Kwiatkowski F, et al. Cellular Expression of Cyclooxygenase, Aromatase, Adipokines, Inflammation and Cell Proliferation Markers in Breast Cancer Specimen. PLoS One 2015;10:e0138443 [Crossref] [PubMed]
- Subbaramaiah K, Morris PG, Zhou XK, et al. Increased levels of COX-2 and prostaglandin E2 contribute to elevated aromatase expression in inflamed breast tissue of obese women. Cancer Discov 2012;2:356-65. [Crossref] [PubMed]
- Qiu X, Cheng JC, Chang HM, et al. COX2 and PGE2 mediate EGF-induced E-cadherin-independent human ovarian cancer cell invasion. Endocr Relat Cancer 2014;21:533-43. [Crossref] [PubMed]
- Plewka A, Madej P, Plewka D, et al. Immunohistochemical localization of selected pro-inflammatory factors in uterine myomas and myometrium in women of various ages. Folia Histochem Cytobiol 2013;51:73-83. [Crossref] [PubMed]
- Ke X, Dou F, Cheng Z, et al. High expression of cyclooxygenase-2 in uterine fibroids and its correlation with cell proliferation. Eur J Obstet Gynecol Reprod Biol 2013;168:199-203. [Crossref] [PubMed]
- Comunoğlu NU, Durak H, Comunoğlu C, et al. Expression of cyclooxygenase-2, c-kit, progesterone and estrogen receptors in uterine smooth muscle tumors: differential diagnosis. APMIS 2007;115:726-35. [Crossref] [PubMed]
- Park SB, Jee BC, Kim SH, et al. Cyclooxygenase-2 inhibitor, celecoxib, inhibits leiomyoma cell proliferation through the nuclear factor κB pathway. Reprod Sci 2014;21:1187-95. [Crossref] [PubMed]
- Thill M, Fischer D, Kelling K, et al. Expression of vitamin D receptor (VDR), cyclooxygenase-2 (COX-2) and 15-hydroxyprostaglandin dehydrogenase (15-PGDH) in benign and malignant ovarian tissue and 25-hydroxycholecalciferol (25(OH2)D3) and prostaglandin E2 (PGE2) serum level in ovarian cancer patients. J Steroid Biochem Mol Biol 2010;121:387-90. [Crossref] [PubMed]
- Thill M, Fischer D, Hoellen F, et al. Prostaglandin metabolising enzymes and PGE2 are inversely correlated with vitamin D receptor and 25(OH)2D3 in breast cancer. Anticancer Res 2010;30:1673-9. [PubMed]
- Miyashita M, Koga K, Izumi G, et al. Effects of 1, 25-Dihydroxy Vitamin D3 on Endometriosis. J Clin Endocrinol Metab 2016;101:2371-9. [Crossref] [PubMed]
- Wang Q, He Y, Shen Y, et al. Vitamin D inhibits COX-2 expression and inflammatory response by targeting thioesterase superfamily member 4. J Biol Chem 2014;289:11681-94. [Crossref] [PubMed]


