
An investigative study of factors affecting breast cancer patients’ preference of extended endocrine therapy
Introduction
Breast cancer is one of the most common malignancies in women worldwide and is the leading cancer-related cause of death in women (1,2). The incidence of breast cancer in China continues to rise in the past decades (3,4). More than half of the cases are hormone receptor (HR)-positive (5,6). Five years of adjuvant endocrine therapy (AET) for HR-positive breast cancer patients has been the mainstay for several decades. But, unfortunately, the recurrence risk of breast cancer does not decrease beyond the initial 5 years of treatment. Several clinical trials have emerged to support extended endocrine therapy (EET) to prolong disease free survival and to reduce cancer mortality (7-9). Ten years of endocrine therapy have been more and more frequently recommended for modest and high recurrent risk patients by therapeutic guidelines in recent years. Even though the efficaciousness of initial and EET has been verified, it is a common phenomenon that many patients do not receive these treatments as prescribed (10). Both early discontinuation and non-adherence to endocrine therapy have been proved to be associated with increased mortality (11,12).
For patients with poor adherence, the extension of treatment duration beyond 5 years is hardly realistic. The improvement of adherence rate for those patients is the first step to decrease mortality. For patients with good adherence, EET is practical and feasible. With the consideration of side effect of endocrine therapy and economic and psychological burden of cancer therapy, it is conceivable that not all the patients who finished 5 years of therapy are willing to extend treatment duration. The decision-making of EET should not only focus on the cancer biology alone but also include treatment side effects and patients’ preference, but little information has been reported whether the patients are willing to extend the therapy. The aim of our study was to assess the patients’ preference of EET and relevant factors in a population of Chinese women with HR-positive breast cancer in order to look for modifiable factors to increase compliance.
Methods
Patient and methods
The study was approved by the institutional review boards of Peking Union Medical College Hospital (PUMCH). All patients in this study provided their signed informed consent.
An investigational study was performed on patients attending breast cancer follow-up clinics in PUMCH (a large tertiary integrated medical Centre and a university teaching hospital) over a 3-month period between May 2017 and July 2017. Within our department, each breast cancer patient attends the follow-up clinic biannually for clinical review for the first 2-year post-operatively and then annually after 2 years. Almost all the patients with HR-positive tumors were prescribed therapeutic AET unless the patient has a contraindication for medication. Choice of hormonal therapy is generally depended on the patient’s menopausal status and tumor recurrence risk. Premenopausal and perimenopausal patients with low or intermediate risk tumors tend to be prescribed tamoxifen or toremifene and patients with high risk may tend to be recommended adding ovarian suppression or ablation. Majority of the postmenopausal patients are offered an aromatase inhibitor (AI) initially for at least 5 years and some change to tamoxifen or toremifene after 2 or 3 years’ AI therapy.
Eligible participants were women, diagnosed with HR positive breast cancer, who completed surgery, chemotherapy, and radiation if necessary, had been taking endocrine therapy for at least 6 months with good adherence, and able to complete the questionnaire. A total of 256 women meeting the inclusion criteria were invited to participate by outpatient nurse at their routine follow-up clinics. They all received an introductory letter, an informed consent, a questionnaire and self-adhesive envelope. Each consenting patient completed the written questionnaire providing self-reported information regarding socio-demographics, clinicopathologic features, surgical and adjuvant treatment, household finances, medical insurance, medication adherence and preference of EET. Signature in the questionnaire was preferred to double-check the accuracy of patients’ clinicopathologic features. The completed questionnaire was personally enclosed in the envelope by the patient. All the envelopes were uniformly opened by researchers at data statistics phase. Two hundred and seven women responded to the survey (response rate 80.9%). Of these, twenty-eight were excluded from the sample because of the incomplete information. The final sample included 179 respondents.
Clinicopathologic features of these patients were collected from their medical records including tumor size, histologic grade, axillary lymph node status, TNM (tumor/node/metastasis) classification, estrogen receptor (ER) and progesterone receptor (PR) status (% staining by immunohistochemistry, IHC), HER2/neu status [IHC staining score (0, 1+, 2+, 3+) and when available FISH ratio] and Ki-67 index.
The clinicopathologic surrogate definition for liminal A subtype included all of ER positive, PR cut-point of ≥20%, HER2 negative and Ki-67 <14%. The tumor with ER positive, HER2 negative, PR <20% and/or Ki-67 ≥14% was defined as luminal B1 and the tumor with ER positive and HER2 positive was defined as luminal B2 subtype.
We categorized patients as being adherent if the ratio of total days covered by the medication divided by the days needing the medication (self-reported), i.e., the medication possession ratio (MPR), was greater than or equal to 80%, a definition commonly used in the adherence literature (13-15).
Statistical analysis
Statistical analyses were performed using SPSS (version 19.0). Categorical variables were compared via Chi-squared and Fisher’s Exact tests. Multivariate logistic regression analysis was used to analyze the association between EET preference with patient clinical and demographic factors. A P value of <0.05 was considered to indicate a significant result.
Results
Participants
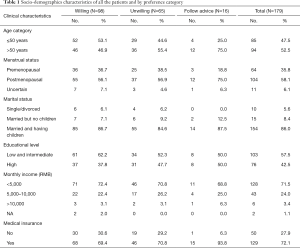
The participants’ median age was 51 years (range, 26–84 years). Eighty-five (47.5%) participants were aged ≤50 years, and 94 (52.5%) were aged >50 years (Table 1). Most women were postmenopausal (104 of 179; 58.1%), married and having children (154 of 179; 86.0%), and had completed an intermediate-level (senior middle school) education (103 of 179; 57.5%). Seventy-two percent (128 of 179) participants’ monthly income was less than 5,000 RMB, and 72.1% (129 of 179) had medical insurance. Among all the 179 patients, 98 (54.7%) were willing to extend endocrine therapy to 10 years, 65 (36.3%) were unwilling and the remaining 16 patients (8.9%) would like to follow the doctor’s advice. All the socio-demographics characteristics did not significantly differ among the three groups (P>0.05).
Full table
Clinicopathology and treatment
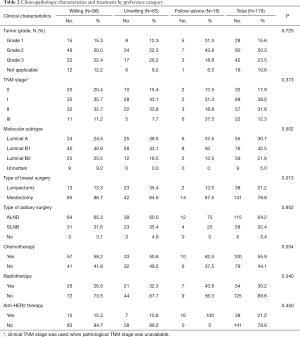
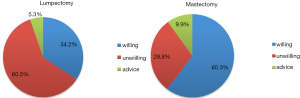
The tumor grade, TNM stage and molecular subtype in the three groups did not significantly differ (Table 2) (P>0.05). The type of breast surgery was associated with the preference of EET (Figure 1) (P=0.013), but the type of axillary surgery was not (P=0.952). Chemotherapy, radiotherapy and anti-HER2 therapy did not differ significantly in the three groups (P>0.05). In the multivariate logistic regression analysis to determine predictors of EET preference, mastectomy was associated with more willingness to EET (P=0.001, OR=4.010, logistic back). There were 141 mastectomy patients, being 60% more likely to extend therapy and 30% more likely to be unwilling to EET. The percentage of unwillingness in lumpectomy patients was 60% instead.
Full table
Medication behavior
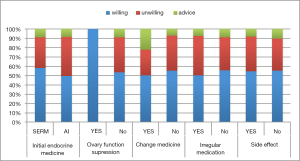
One third of the participants had received endocrine therapy for more than 2 years (58 of 179) in our study. The medication duration (≤2 vs. >2 years) was not an influence factor for EET preference (P=0.341). More than 60% participants took SERM as initial therapy. Only 5 patients in this study received OFS treatment and all of them were willing to EET. About on tenth of the patients (18 of 179) changed their medicine during the endocrine treatment. Twenty-six (14.5%) participants had irregular medication and 141 (78.8%) participants reported drug side effect. The proportion of EET preference did not significantly differ in patients with or without medicine change, irregular medication and side effect (Figure 2) (P>0.05).
Discussion
In this investigational study of women with good adherence to AET, we found that approximately 55% participants were willing to extend endocrine therapy to 10 years, 36% were unwilling and 9% would like to follow the doctor’s advice. Furthermore, we found that, all the socio-demographics and clinicopathologic characteristics did not significantly differ among the three groups and the rates of chemotherapy, radiotherapy and anti-HER2 therapy were similar between groups. We found that the type of breast surgery was the only factor associated with the preference of EET with approximately 60% mastectomy patients willing to EET and 60% lumpectomy patients unwilling. We were a bit surprised to find that the EET preference did not significantly differ in patients with or without medicine change, irregular medication and side effect.
To our knowledge, this is the first study to investigate the patients’ preference for EET in patients with good adherence to AET. Patients who had been taking endocrine therapy for at least 6 months with good adherence were included in our study, ensuring that each patient had sufficient understanding and experience of endocrine therapy and sufficient compliance up to now.
Although AET has been demonstrated by multiple clinical trials to be an efficacious treatment for breast cancer patients (7-9,16), non-adherence rates in this group remain high (10,12,17,18). Previous study (10) showed that the proportion of patients who continued therapy decreased year by year. Of the patients who continued, adherence rate was less than 80% in the first year. Given the great efficacy of AET and the realization that poor adherence to treatment may reduce the beneficial effect, identification of causes of low adherence, especially modifiable causes, is of critical importance for such patients (19). It is appropriate and feasible to discuss about EET only if the patient’s compliance is assured.
As we all know, endocrine therapy is greatly different from chemotherapy and radiotherapy. Comparing with the latter, the side effects of endocrine therapy are more long-lasting and more difficult to cope with even if the side effects are considered milder. Adherence to the treatment for 5 years has already been a real difficult task for most patients, and the completion of 5 years of treatment does not mean that the patients are willing to undergo 10 years of treatment with the consideration of side effects and inconvenience. So, it is unsurprising that more than one third of participants in our study were unwilling to extend treatment. And it is conceivable that the proportion of EET willingness would be much less if the study population was extended to patients with poor compliance.
The patient, physician, and clinicopathological factors contributing to a decision for EET are complicated. The effective communication between physician and patients are of great importance to treatment decision-making. Understanding the individual patient’s values and goals, explaining carefully the benefits and adverse effects of the treatment are paramount to providing a shared decision-making process.
This survey focused on the patients’ willingness of EET which depended totally on the patients’ medicine taking experience and individual anticipation of treatment benefits. The extents by which patients are informed about the estimated benefit and whether the patients had fully understood were not evaluated in the survey although the possible treatment benefit were described in the questionnaire by listing the percentage of survival benefit from published clinical trials. Perhaps further face-to-face communication may improve the patient's expected benefit from the treatment and improve the EET willingness proportion. More relevant research will be conducted to verify the hypothesis.
Communication is on one hand, different patients having different treatment anticipation is on the other hand. In the study about the patient’s preference for initial AET, some women judged small benefits sufficient to make their AET worthwhile, but many women required larger benefits (20).
Our study identified that the type of breast surgery was the only key factor associated with EET preference. Patients receiving lumpectomy were more unwilling to EET. Compared with mastectomy, breast conserving therapy did not affect overall survival or time to distant metastases, but resulted in worse local control (18,21). EET has been showed to decrease the recurrence event including local-regional recurrence (16,22) . From the local recurrence point of view, it is more necessary for breast conserving patients to receive EET. But, comparing with breast conserving patients, mastectomy patients concern more about recurrence and mortality. It is possible that they might judge smaller benefits necessary to make EET worthwhile and more willing to extend treatment. However, the breast conserving patients have higher requirements for body shape and sex life, and extended treatment means that the body changes caused by drugs and vaginal dryness are going to be further prolonged. Although side effect was not shown to be an influence factor for EET decision-making in our study, it is inappropriate to ignore the fact that patients will take toxicity into account when making decisions. And it is worth attention that our study only included patients with good adherence but not the nonadherer, which means the side effects were well tolerated by this patient group at the moment. But the well tolerance in the past years does not equal to the compliance in the future.
Considering the treatment-associated toxicity is often a major barrier to the full application of effective cancer treatment (17), the balance of treatment benefit anticipation and long-lasting side effects may be a key factor for patients to make decision whether it is worthwhile to extend therapy. In the recent years, multigene assays has been able to assess long-term relapse risk and therefore might be helpful for patients’ decisions on duration of AET (23).
Previous study showed that younger women were at high risk of AET nonadherence (10). In our study, younger participants (≤50 years) seemed a bit more likely to EET with no significant statistic difference. Although EET is an extension of AET from a therapeutic point of view, the preference and compliance for treatment might be different especially when we enrolled only so-called adherer in our study.
In the previous studies about the compliance of AET, Asian/Pacific Islander women were significantly less likely than other racial/ethnic groups to discontinue therapy, but there was no difference in rates of nonadherence (10). Other studies showed that Asian/Pacific Islander women including Chinese women were significantly more likely to undergo mastectomies than white women (24,25). Our results are consistent with these studies that approximately 80% participants choose mastectomy instead of breast conserving therapy. Different treatment preference across different populations should be taken into account when we interpret research findings.
There are several limitations to our study. First, we defined adherence by patient self-report. It might be different from real world situation. The self-reported adherence were usually higher than actuality (26). The study results might be influence by this bias. The real preference for EET might be lower than we expected. Another limitation was the relatively short medication duration of the enrolled patients. Although the medication duration (≤2 vs. >2 years) was not an influence factor for EET preference, the real decision-making for EET might be changed after completing 5 years’ AET. Finally, our trial is an investigational study without interference in one breast cancer therapeutic center. The research population was restricted and the sample size was small.
We found that more than one third of the enrolled patients with good AET adherence were unwilling to EET. Among the demographic and clinicopathologic characteristics and the medication behavior, the type of breast surgery was the only influence factor for EET preference. Further investigation is critical to identify interventions, for example face-to-face communication, regular interview, timely management for drug side effect, to help more patients comply with the full course of adjuvant HT, especially for breast conserving patients. All the interventions in improving compliance of AET and EET have the potential to improve breast cancer survival.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.02.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the institutional review boards of Peking Union Medical College Hospital (PUMCH). All patients in this study provided their signed informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Fan L, Strasser-Weippl K, Li JJ, et al. Breast cancer in China. Lancet Oncol 2014;15:e279-89. [Crossref] [PubMed]
- Millis JM. Historical perspective on the importance of the Milan criteria. Hepatobiliary Surg Nutr 2016;5:485-7. [Crossref] [PubMed]
- Kamal AH, Loprinzi CL, Reynolds C, et al. Breast medical oncologists' use of standard prognostic factors to predict a 21-gene recurrence score. Oncologist 2011;16:1359-66. [Crossref] [PubMed]
- Ishizawa T, Saiura A, Kokudo N. Clinical application of indocyanine green-fluorescence imaging during hepatectomy. Hepatobiliary Surg Nutr 2016;5:322-8. [Crossref] [PubMed]
- Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 2013;381:805-16. [Crossref] [PubMed]
- Gray RG, Rea D, Handley K, et al. aTTom: long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6,953 women with early breast cancer. J Clin Oncol 2014;31:abstr 5.
- Jin H, Tu D, Zhao N, et al. Longer-term outcomes of letrozole versus placebo after 5 years of tamoxifen in the NCIC CTG MA.17 trial: analyses adjusting for treatment crossover. J Clin Oncol 2012;30:718-21. [Crossref] [PubMed]
- Hershman DL, Kushi LH, Shao T, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol 2010;28:4120-8. [Crossref] [PubMed]
- Barron TI, Cahir C, Sharp L, et al. A nested case-control study of adjuvant hormonal therapy persistence and compliance, and early breast cancer recurrence in women with stage I-III breast cancer. Br J Cancer 2013;109:1513-21. [Crossref] [PubMed]
- Hershman DL, Shao T, Kushi LH, et al. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat 2011;126:529-37. [Crossref] [PubMed]
- Karve S, Cleves MA, Helm M, et al. Good and poor adherence: optimal cut-point for adherence measures using administrative claims data. Curr Med Res Opin 2009;25:2303-10. [Crossref] [PubMed]
- Osterberg L, Blaschke T. Adherence to medication. N Engl J Med 2005;353:487-97. [Crossref] [PubMed]
- Piardi T, Lhuaire M, Memeo R, et al. Laparoscopic Pringle maneuver: how we do it? Hepatobiliary Surg Nutr 2016;5:345-9. [Crossref] [PubMed]
- Goss PE, Ingle JN, Pritchard KI, et al. Extending Aromatase-Inhibitor Adjuvant Therapy to 10 Years. N Engl J Med 2016;375:209-19. [Crossref] [PubMed]
- Murphy CC, Bartholomew LK, Carpentier MY, et al. Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: a systematic review. Breast Cancer Res Treat 2012;134:459-78. [Crossref] [PubMed]
- McCowan C, Shearer J, Donnan PT, et al. Cohort study examining tamoxifen adherence and its relationship to mortality in women with breast cancer. Br J Cancer 2008;99:1763-8. [Crossref] [PubMed]
- Kimmick G, Edmond SN, Bosworth HB, et al. Medication taking behaviors among breast cancer patients on adjuvant endocrine therapy. Breast 2015;24:630-6. [Crossref] [PubMed]
- Duric VM, Fallowfield LJ, Saunders C, et al. Patients' preferences for adjuvant endocrine therapy in early breast cancer: what makes it worthwhile? Br J Cancer 2005;93:1319-23. [Crossref] [PubMed]
- Litiere S, Werutsky G, Fentiman IS, et al. Breast conserving therapy versus mastectomy for stage I-II breast cancer: 20 year follow-up of the EORTC 10801 phase 3 randomised trial. Lancet Oncol 2012;13:412-9. [Crossref] [PubMed]
- Jakesz R, Greil R, Gnant M, et al. Extended adjuvant therapy with anastrozole among postmenopausal breast cancer patients: results from the randomized Austrian Breast and Colorectal Cancer Study Group Trial 6a. J Natl Cancer Inst 2007;99:1845-53. [Crossref] [PubMed]
- Knauer M, Filipits M, Dubsky P. Late recurrences in early breast cancer: for whom and how long is endocrine therapy beneficial? Breast Care (Basel) 2014;9:97-100. [Crossref] [PubMed]
- Goel MS, Burns RB, Phillips RS, et al. Trends in breast conserving surgery among Asian Americans and Pacific Islanders, 1992-2000. J Gen Intern Med 2005;20:604-11. [Crossref] [PubMed]
- Li J, Zhang BN, Fan JH, et al. A nation-wide multicenter 10-year (1999-2008) retrospective clinical epidemiological study of female breast cancer in China. BMC Cancer 2011;11:364. [Crossref] [PubMed]
- Ziller V, Kalder M, Albert US, et al. Adherence to adjuvant endocrine therapy in postmenopausal women with breast cancer. Ann Oncol 2009;20:431-6. [Crossref] [PubMed]


