The modern concept of breast conserving surgery
Introduction
The achievement on the biological behaviour and natural history of breast cancer demonstrates the same effectiveness and oncological safety of breast conserving surgery (BCS) followed by radiotherapy than mastectomy.
The goal of BCS for patients with early-stage breast cancer is the complete removal of the tumor keeping sufficient surgical margins and maintaining at the same time the natural shape and appearance of the breast.
In the recent past, many women just thought to get their lives safe. So, they accepted every breast deformities in order to be healthy.
Nowadays, it becomes necessary for breast surgeons to offer them a variety of surgical options so they can remove the cancer from their body and from their mind too.
Since 1990s, some visionaries tried to change the traditional way to remove breast cancer.
Audretsch first in 1987 (1), Gabka and Bohmert after (2), introduced the term “oncoplastic surgery” (OPS) for describing the hybrid approach that allows to avoiding mastectomy by wide tumour excision associated with partial breast reconstruction.
Oncoplastic breast OPS combines oncologic principles with plastic surgical techniques to ensure an oncologically safe resection and generate satisfying cosmetic results.
As of today, the BCS, thanks to oncoplastic techniques, allows patients who would require mastectomy to obtain clear margins minimizing potential complications.
The general approval and diffusion of the OPS require surgeons to become familiar with the different techniques and indications, to get it more safe and efficient.
The OPS demands the experience and the knowledge in the oncological aspects of a general surgeon and the ability in the breast reconstruction of a plastic surgeon. According to above the clinical practice of OPS has been slow, but today it includes an extensive range of breast-conserving surgical techniques.
Classifications
Two different approaches can be used by oncoplastic breast surgeons based on reconstruction techniques following chosen BCS.
Volume displacement techniques
Firstly, volume displacement techniques may include adjacent tissue rearrangement (simply by undermining and closure of the defect) or mastoplastic procedures.
Adjacent tissue rearrangement is the most common procedure for filling wide resection defect.
The key factors in adjacent tissue rearrangement techniques are the following:
- Accurate decision of skin incision: aesthetic incision along areola border or inframammary line;
- Extensive subcutaneous undermining: tumor resection and glandular redistribution;
- Nipple-areola complex (NAC) undermining, deepithelization and NAC repositioning;
- Glandular reapproximation: creation of glandular flaps for closing the defect.
Mammaplasty techniques enable reconstruction of surgical defects with parenchymal flaps through the superior or the inferior pedicle approach.
When wide resection of tumors located in the lower quadrants of the breast leads to an extensive volume loss, the superior pedicle approach is preferable.
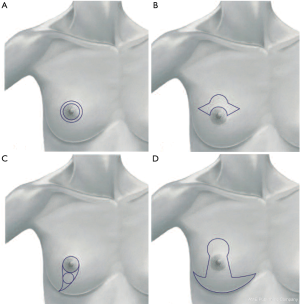
The inferior pedicle approach allows reconstruction of surgical defects in the upper pole of the breast (Figure 1).
Variations and adaptations of the superior and inferior pedicle approaches have recently been described.
There are clinical situations in which alternative techniques, such as the “Grisotti” technique, the “round block” and “batwing” approach, can be adapted to enable resection of tumor (Figure 2) (3-9).
Volume replacement techniques (VRT)
Secondly, VRT reconstruct the surgical cavity by replacing the volume of tissue removed with autologous tissue from an extramammary site. It’s possible to maintain the shape and the original size of the breast achieving cosmetic results without any contralateral surgery.
Many oncoplastic techniques have been describing, by using of fascio-cutaneous flaps, myocutaneous local flaps, pedicled perforator flaps and free flaps.
When the defect ranges from 10% to 30% of the breast volume, the most commonly used flap, a myocutaneous flap, is the latissimus dorsi musculocutaneous flap (Figure 3); lateral, central and inferior defects are filled with latissimus dorsi and overlying skin.
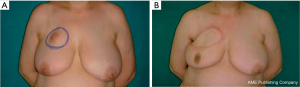
There have been different techniques described by which the latissimus dorsi muscle flap can be harvested. The traditional technique includes a posterolateral thoracic incision, whereas the more modern technique utilizes an endoscope. When the skin above muscle needs to be preserved we can reach latissimus dorsi through the breast by using the endoscope (10,11).
Another method of harvesting the latissimus dorsi is as a mini-flap. The advantage of the mini-flap is that variable amounts of the latissimus dorsi muscle can be harvested based on the volume requirements of the breast. The flap is generally harvested through an anterolateral breast incision that is used for the resection as well.
There are three flaps: the thoracodorsal artery perforator (TDAP) flap, the lateral thoracic flap, and the intercostal perforator flap (12).
Other classifications
Urban et al. (13-15) developed another classification based on 3 distinct levels that require specific competence in plastic surgery.
Hoffmann et al. (16) proposed a complex classification system capable of accommodating, on the basis of surgical complexity, any major oncological, oncoplastic or reconstructive procedure used in the surgical treatment of primary and locally recurrent breast cancer.
Clough et al. (17,18) proposed a classification based on the amount of tissue excised and the relative level of surgical difficulty (this classification concerns the volume displacement procedures but does not include the VRT):
- Level I approach in which less than 20% of breast volume is excised and it is no required skin resection; there are 6 steps for level I (skin incision, skin undermining, NAC undermining, full-thickness excision, glandular reapproximation, deepithelialization and NAC repositioning);
- Level II approach in which up to 50% of breast volume is excised and therapeutic mammoplasties with extensive skin excision and breast reshaping are performed; to simplify the selection of the appropriate technique, Clough et al. devised an Atlas based on tumor location; this atlas provides one or two surgical techniques for each tumor location.
When can we use OPS?
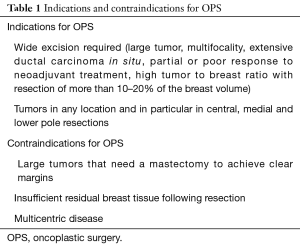
Main goals of OPS are achieving safe margin resection while preserving breast contour and balanced cosmetic result (Table 1).
Full table
Wide resection of more than 20% of glandular volume, extensive ductal carcinoma in situ, multifocality or cancers located in central, medial or lower pole are all perfectly managed by oncoplastic procedures.
We can’t use OPS when we can’t ensure oncological radicality:
- Large tumours and/or multicentric tumours (they may benefit from a mastectomy);
- Inflammatory tumours (for the same past reason);
- Previous radiotherapy;
- Prior augmentation mammoplasty;
- Multiple comorbidities or patients that are active smokers (they are not ideal candidates for some complex oncoplastic techniques).
Selection criteria of the technique
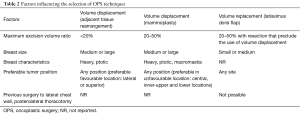
There are controversy, scrutiny, and criticism about the selection criteria for oncoplastic techniques. Because the different indications for every oncoplastic techniques, various algorithms have been conceived to assist with the decision process (19). The choice is generally based on cancer characteristics (size and location), extent of resection, breast characteristics (size, shape and glandular density), previous surgery and the patient’s expectations (5,17,20-25) (Table 2).
Full table
Patients with large-medium sized breasts, ptosis and dense glandular tissue, <20% breast volume excision, tumor localized in lateral and superior quadrants (5,17,18,20) are candidates to volume displacement techniques with adjacent tissue rearrangement.
Patients with large-medium sized breasts, 20–50% breast volume excision, tumor localized in any site of the breast but especially for unfavourable location as central, inner-upper and lower quadrants are candidates for volume displacement techniques with reduction mammoplasty.
Patients with heavy, ptotic breasts and symptomatic macromastia are appropriate candidate to bilateral reduction mammaplasty techniques, they also will benefit physically from the use of a bilateral procedure.
There are some cases in which the VRT cannot be employed: lack of latissimus dorsi muscle and if the vascular pedicle has been injured or tied (for example in a previous axillary surgery or thoracotomy) (20,26-28).
These techniques are suitable for patients that need 20–50% breast volume excision, tumor localized in any site, small-medium sized breasts and minimal ptosis.
On the other hand, we can use VRT for patients who prefers to avoid mastectomy or contralateral surgery, or who cannot accept to lose the volume associated with volume displacement techniques.
Preoperative planning
It is very important for psychological and emotional aspects of cancer patient to solve her problem in one time. So, we need to make a proper and strong preoperative planning by reviewing the patient’s diagnosis, pathology, imaging, recurrence risk, eventual contralateral treatment, the need for radiotherapy.
Then we can imagine and draw the best surgical technique that fit on the breast patient like a dress that emphasize woman beauty.
This approach requires the work of multidisciplinary team composed by breast surgeons, plastic surgeons, radiologists, radiotherapists, oncologists, pathologists and psychologists.
They all do their best to treat the patient as the only one.
The available options are explained to patient, highlighting the advantages, disadvantages, and technical challenges of each procedure.
Different oncoplastic techniques can be used for cancers located in the various quadrants of the breast (Table 3).

Full table
Periareolar lesions
Oncoplastic volume displacement techniques provide excellent outcomes in the treatment of periareolar lesions. Donut mastopexy or batwing mastopexy is preferable for breasts with minimal or moderate ptosis.
Reduction mammaplasty pattern is suitable for breasts with severe ptosis or redundant skin.
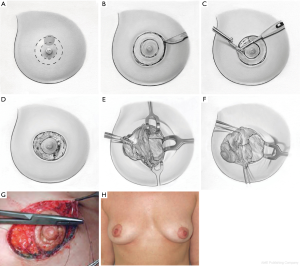
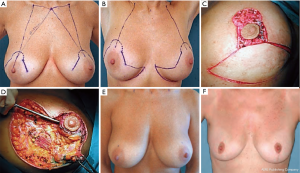
Donut mastopexy
The donut mastopexy technique enables comfortable access to different lesions in the periareolar region compared with traditional breast-conserving approaches. Two concentric circles with different diameters are designed around the nipple (Figure 4A). The areolar skin is stretched only mildly when the inner circle is designed to avoid the potential for the final areolar diameter being too small. The measure of the inner round is usually from 4 to 4.5 cm, depending on the size of the breast. The diameter of the outer round should not exceed the diameter of the original areola by more than 20 to 25 mm to limit widening the circumareolar scar or severe flattening of the breast.
The incision of the inner circle corresponds to the new border of the areola (Figure 4B).
The outer round is incised, whereas the donut of skin between the two rounds is excised. Through this incision, any periareolar lesion can be easily accessed (Figure 4C,D). The resection of the breast parenchyma can be performed through a wider incision that extends to the pectoralis fascia. In this way the resection is performed with a better control than the conventional conservative skin incisions (Figure 4E,F).
The breast can be reshaped suitably by displacing the residual parenchyma. Generally, we have to separate the residual gland of the pectoralis fascia using electrocautery, paying attention to reduce the perforating vessels that are dissected to avoid interference with the blood supply to the residual glandular tissue. After hemostasis is achieved, the residual breast gland is reapproached to enable a natural-appearing breast. Sutures are applied in the deep portion of the residual parenchyma, just above the muscle fascia, to fix the posterior edges in their new position. We currently use 3-0 Vicryl sutures for this purpose and 4-0 absorbable intradermal sutures. Sometimes, in particular cases, a purse-string suture is applied to reduce the dimension of the larger circle. Then it is sutured to the new border of the areola, so at the end of the procedure just a periareolar scar remains (Figure 4G). The patient is shown 6 months after surgery. Only a periareolar scar is visible (Figure 4H).
We can plan the final NAC position by drawing preoperatively the outer circle in a manner way.
If the two circles are concentric, the NAC position remain the same; if the outer circle is centered around a point located higher than the existing nipple, the NAC can be elevated slightly as a consequence of the procedure.
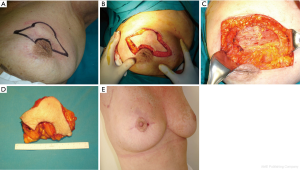
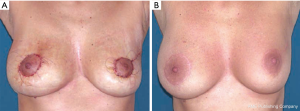
Batwing mastopexy
When cancers are located in the upper periareolar region of the breast, the batwing mastopexy technique is excellent, particularly when the lesions are superficial; a plentiful skin removal overlying the lesion can increase the safety. Two semicircles are designed, one on the border of the areola and one 20 to 25 mm above it, and connected with angled wings on each side of the areola (Figure 5A). The skin incisions should be drawn with the patient in stand position. The areolar semicircle is incised first, followed by the upper semicircle and the wings (Figure 5B). A full-thickness lumpectomy is performed (Figure 5C,D), and the residual parenchyma is separated of the pectoralis fascia, allowing defect remodelling and sufficient tissue advancement.
The procedure allows of the oncologic control of cancers located superficially. At the end of the procedure may result some uplifting of the NAC without significant asymmetry.
The patient is shown 4 months after surgery (Figure 5E).
Reduction mammaplasty
The resection of the tumor with wide clear margins can be easily obtained either with an inferior or superior pedicled flap, to recreate a normally shaped breast and to shift the NAC to an appropriate position.
Central retroareolar lesions
Recently, as an alternative to a total mastectomy, several breast-conserving oncoplastic procedures have been utilized for central tumors involving the retroareolar region or for Paget disease. All of these techniques include a total tumor excision with the complete NAC and the corresponding underlying cylinder of the gland down to the pectoralis fascia. The central defect is restored with a simple purse-string suture, linear sutures, or skin-parenchyma flaps. We usually use the Grisotti technique, because it offers excellent cosmetic results.
Grisotti technique
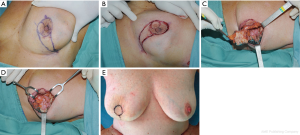
A circle is drawn along the borders of the areola with patient in sitting position. Another circle is drawn below the areola, and lines from the medial and lateral sides of the upper circle are connected laterally on the inframammary fold (Figure 6A). Incisions are made along the drawings, and the skin below the areola is excised, with the exception of the skin included in the lower circle.
The NAC and the underlying tumor is totally removed down to the pectoralis fascia (Figure 6B,C,D). A new areola is created using the skin-gland flap, mobilized from the inferior lateral pole of the residual gland. The flap is incised medially down to the pectoralis fascia and separated from the latter to allow adequate rotation and advancement. It is sutured to the gland stump superiorly to give adequate projection to the tip of the breast mound, and the circular area of preserved skin is sutured to replace the excised areola.
It is very important to reduce the ischemic injury risk of the neoareola, paying attention to prevent excessive devascularization of the skin-gland flap. At the end of the procedure, the breast may be slightly smaller than the contralateral one, but it preserved a good shape.
The patient is shown 2 weeks after surgery (Figure 6E). The nipple reconstruction can be performed immediately, if the patient prefers, or at a later stage, with tattooing of the areola. Contralateral breast remodeling is required if major asymmetry occurs.
Lesions located in the lower quadrants
When standard lumpectomy techniques are used to treat tumors located in the lower region of the breast, cosmetic outcomes could be unsatisfying.
Downturning of the NAC and/or introflection of the lower pole is often seen with these procedures. We prefer reduction mammoplasty pattern in these kind of lesions, that may allow large amounts of breast tissue to be resected, even in small breast, with excellent cosmetic outcomes and wide surgical margins.
Reduction mammaplasty
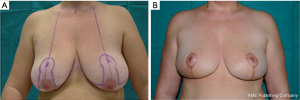
A vertical pattern, an L-shaped pattern, or a keyhole pattern incision may be used. The reduction pattern choice is made based on the ptosis features and the amount of breast parenchyma to be resected. For symmetry, the same reduction pattern is performed contralaterally (Figure 7).
The vertical or J-scar patterns is a very versatile pattern in the setting of breast ptosis. It is not only indicated for resection of a tumor located in the central lower quadrant for which a straightforward resection like the breast reduction technique is indicated.
A tumor located in the medial or lateral lower quadrant, as well as a tumor located in the upper quadrants can benefit from this pattern. In case of a tumor in the lower quadrant the resection is performed through the inferior quadrant incision and for a tumor in the upper quadrant the resection is performed through the periareolar incision. After resection, the lower quadrant pattern vertical or J-scar pattern designed is deepithelialized and an inferior based adipoglandular flap is raised and displaced at the level of the resection to improve the shape. The flap is usually suspended with 1 to 2 stitches of 0 Vicryl to the pectoralis major muscle and approximated with 2-0 Vicryl to the surrounding breast parenchyma.
Example: right breast quadrantectomy in the upper-lateral quadrant using J-scar oncoplastic technique with NAC superior pedicle and contralateral symmetrization (Figure 8A,B).
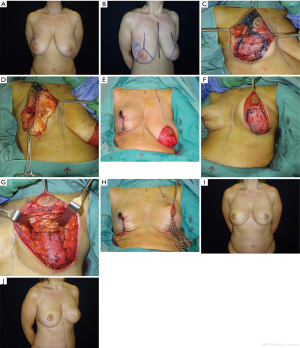
Intraoperative sequence of the oncoplastic procedure, after tumor resection (Figure 8C), inferior pole flap dissected to fill the upper defect (Figure 8D) and temporarily based (Figure 8E).
Contralateral symmetrization with inferior pole dermoglandular flap for autoaugmentation (Figure 8F,G,H). The patient is shown 9 months after surgery (Figure 8I,J).
As for the keyhole pattern, the skin marking are as follows. A mark is made in the center of the sternal notch; each clavicle is marked 6 cm laterally from this sternal mark (Figure 9A). A straight line is drawn from each clavicular mark to the nipple of the breast below (Figure 9B).
The center of the proposed nipple location is sited on this line, from 19 to 23 cm from the sternal notch mark, depending on the size of the patient (Figure 9A). A semicircle with a 5 cm diameter is drawn, centered on the new nipple location. Radial lines 6 cm long are designed from the lower half of the circle and connected in straight lines to markings previously made on the inframammary creases (Figure 9B). Medially, these lines should connect about 1 cm from the midline and should never reach the medial drawings of the contralateral breast. The lateral end of the inframammary crease is not marked on its natural ending (because it extends too far laterally and too low), but rather is crossed superiorly on the midaxillary line to terminate 2 to 3 cm superior to the crease. This end is connected with a straight line to the inferior end of the lateral wing. The skin markings are progressively incised, and the lesion is totally removed with the overlying skin (Figure 9C). The parenchymal excision is extended down to the fascia of the pectoralis major muscle. A superior pedicle flap is created to mobilize the NAC (Figure 9D).
The gland excision can be included more lateral or medial portions of the parenchyma in tumors localized in the inferolateral or inferomedial quadrants. So more extended undermining of either the medial or lateral flap it is required. At the end of the parenchymal excision, the medial and lateral flaps are sutured together to restore the normal shape of the breast, leaving a vertical, L-shaped, or typical inverted-T scar.
The patient is shown preoperatively (Figure 9E) and 6 months after surgery (Figure 9F). The reduction mammaplasty approach is very suitable in women with large and pendulous breasts, because it improves the aesthetic aspect of the gland and can promote the delivery of postoperative radiotherapy.
Lesions located in the upper quadrants
Cancers localized in the upper outer quadrant usually does not necessitate any special reconstruction, and adequate aesthetic results can be realized simply by approximating the residual parenchyma in layers. Sometimes, the tumor excision generates a larger defect, so a kind of reconstruction is required even in the upper outer or upper inner quadrants. In the setting of a large and ptotic breast, a reduction pattern is useful as the tumor resection can be performed from the periareolar approach and the volume can be displaced by mobilizing up an inferior based adipoglandular flap from the lower quadrant.
VRT
When the breast is not large and ptotic and the resection is proportionally wider than the residual breast, volume displacement techniques has limited power to properly correct the defect. In this setting, VRT with the use of locoregional or distant flaps are often needed to properly reconstruct the defect.
As for the locoregional flap, many subtypes of flaps can be constructed on the thoracodorsal artery pedicle, (such as pedicled latissimus dorsi, muscle or musculocutaneous flap), or pedicled adipocutaneous TDAP flap (29).
As the volume is restored, symmetry is usually maintained and contralateral surgery is rarely required.
The cosmetic outcome is generally better when replacement techniques are used to restore a defect in the upper outer quadrant.
Procedures on the contralateral breast
Reshaping the contralateral breast may be included in the treatment planning to improve symmetry and the cosmetic outcome.
The option of a mastopexy or volume reduction of the contralateral breast should be discussed with the patient, particularly younger women and women with large and pendulous breasts.
If the oncoplastic procedure uses mammoplasty patterns, the same pattern should be used for the contralateral surgery.
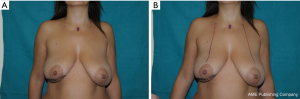
We often use a periareolar mastopexy when the difference between the breasts is not extreme, because it is relatively easy and fast, and it allows the contralateral breast to be elevated a maximum of 2 cm. This patient is shown shortly after a periareolar mastopexy was performed on the right breast simultaneously with an oncoplastic upper external quadrantectomy of the left breast; it was completed to achieve symmetry (Figure 10A). The final result is shown 6 months after surgery (Figure 10B). The design for the concentric mastopexy can be made with the patient in the supine position. Reduction mammaplasty is more long-lasting, but it consents more effective symmetrization, especially when dealing with large, ptotic breasts that need major lifts. In this particular case the markings have to be made with patient in standing position.
During the symmetrization procedures, the surgeon should take the chance to remove any suspicious tissue in the contralateral breast that was seen on the preoperative mammogram. In many series, this has resulted in a 5% detection rate of contralateral subclinical cancers.
Outcomes
The most commonly reported outcomes on studies of OPS are local recurrence, cosmesis and patient satisfaction (30,31). but frequently these studies are retrospective and based on an inadequate number of patients and sometimes only a single surgeon’s experience. Moreover, the methods of assessing cosmesis and patient satisfaction vary considerably. These outcomes have been reported in studies in which the length of follow-up is relatively short, with a median duration of around 5 years. Despite of these limitations, the reported rates of local recurrence and cosmetic failure are within acceptable limits when compared with conventional BCS.
Conclusions
Nowadays, it’s not acceptable in the surgical management of breast cancer not taking care of oncological and cosmetic needs of individual patients.
Oncoplastic preserving surgery is oncologically safe and allows to excise the tumor with negative margins without compromising aesthetic outcome.
Oncoplastic breast surgery can be safely applied in larger tumors, resulting in comparable postoperative complications, resection margins and re-excision rates compared to standard lumpectomy. The excitement related to this procedure comes from reported data that seem to demonstrate a higher oncological safety and better cosmetic efficacy.
Further prospective studies need to be done in order to have longer follow-up time (more than 5 years) and observe overall survival (OS), disease-free survival (DFS), relapse rate (RR) (32).
Acknowledgments
Thanks to Antonia Conti (Medical Illustrator CMI, AMI professional member) that drawn round block black/white pictures.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Cancer Research for the series “Update of Current Evidences in Breast Cancer Multidisciplinary Management”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.01.32). The series “Update of Current Evidences in Breast Cancer Multidisciplinary Management” was commissioned by the editorial office without any funding or sponsorship. GF served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Translational Cancer Research from Nov 2016 to Dec 2018. RM served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Audretsch W. Space-holding technic and immediate reconstruction of the female breast following subcutaneous and modified radical mastectomy. Arch Gynecol Obstet 1987;241:S11-9. [Crossref] [PubMed]
- Gabka CJ, Bohmert H. Future prospects for reconstructive surgery in breast cancer. Semin Surg Oncol 1996;12:67-75. [Crossref] [PubMed]
- Masetti R, Pirulli PG, Magno S, et al. Oncoplastic techniques in the conservative surgical treatment of breast cancer. Breast Cancer 2000;7:276-80. [Crossref] [PubMed]
- Franceschini G, Terribile D, Fabbri C, et al. Progresses in the treatment of early breast cancer. A minireview. Ann Ital Chir 2008;79:17-22. [PubMed]
- Masetti R, Di Leone A, Franceschini G, et al. Oncoplastic techniques in the conservative surgical treatment of breast cancer: an overview. Breast J 2006;12:S174-80. [Crossref] [PubMed]
- Anderson BO, Masetti R, Silverstein MJ. Oncoplastic approaches to partial mastectomy: an overview of volume-displacement techniques. Lancet Oncol 2005;6:145-57. [Crossref] [PubMed]
- Franceschini G, Terribile D, Magno S, et al. Conservative treatment of the central breast cancer with nipple areolar resection: an alternative oncoplastic technique. G Chir 2008;29:23-7. [PubMed]
- Franceschini G, Masetti R, D'Alba P, et al. Conservative treatment with nipple-areolar resection for subareolar breast cancer. Breast J 2006;12:91-2. Erratum in Breast J 2006;12:289. [Crossref] [PubMed]
- Franceschini G, Masetti R, D'ugo D, et al. Synchronous Bilateral Paget’s Disease of The Nipple Associated with Bilateral Breast Carcinoma. Breast J 2005;11:355-6. [Crossref] [PubMed]
- Missana MC, Pomel C. Endoscopic latissimus dorsi flap harvesting. Am J Surg 2007;194:164-9. [Crossref] [PubMed]
- Güemes A, Sousa R, Cachón R, et al. Minimally invasive breast surgery. Breast reconstruction using pure muscular latissimus dorsi flap. Cir Esp 2008;83:85-8. [PubMed]
- Ortiz CL, Mendoza MM, Sempere LN, et al. Versatility of the pedicled thoracodorsal artery perforator (TDAP) flap in soft tissue reconstruction. Ann Plast Surg 2007;58:315-20. [Crossref] [PubMed]
- Urban C, Lima R, Schunemann E, et al. Oncoplastic principles in breast conserving surgery. Breast 2011;20:S92-5. [Crossref] [PubMed]
- Urban CA. Oncoplastic in a pre-paradigm era: a Brazilian perspective in an American problem. Plast Reconstr Surg 2010;125:1839-41. [Crossref] [PubMed]
- de Andrade Urban C. New classification for oncoplastic procedures in surgical practice. Breast 2008;17:321-2. [Crossref] [PubMed]
- Hoffmann J, Wallwiener D. Classifying breast cancer surgery: a novel, complexity-based system for oncological, oncoplastic and reconstructive procedures, and proof of principle by analysis of 1225 operations in 1166 patients. BMC Cancer 2009;9:108. [Crossref] [PubMed]
- Clough KB, Kaufman GJ, Nos C, et al. Improving breast cancer surgery: a classification and quadrant per quadrant atlas for oncoplastic surgery. Ann Surg Oncol 2010;17:1375-91. [Crossref] [PubMed]
- Clough K, Kaufman G, Nos C, et al. Reply to Comments on: Improving Breast Cancer Surgery: A Classification and Quadrant per Quadrant Atlas for Oncoplastic Surgery. Ann Surg Oncol 2011;18:259-60. [Crossref]
- Weber WP, Soysal SD, Fulco I, et al. Standardization of oncoplastic breast conserving surgery. Eur J Surg Oncol 2017;43:1236-43. [Crossref] [PubMed]
- Franceschini G, Magno S, Fabbri C, et al. Conservative and radical oncoplastic approches in the surgical treatment of breast cancer. Eur Rev Med Pharmacol Sci 2008;12:387-96. [PubMed]
- Santanelli F, Paolini G, Longo B. Comments on: Improving Breast Cancer Surgery: A Classification and Quadrant per Quadrant Atlas for Oncoplastic Surgery. Ann Surg Oncol 2011;18: S257-8; author reply S259-60.
- Franceschini G, Terribile D, Magno S, et al. Update in the treatment of locally advanced breast cáncer: a multidisciplinary approach. Eur Rev Med Pharmacol Sci 2007;11:283-9. [PubMed]
- Franceschini G, Terribile D, Fabbri C, et al. Management of locally advanced breast cancer. Minireview. Minerva Chir 2007;62:249-55. [PubMed]
- Delay E, Clough KB. Oncoplastic breast surgery: conclusions and future perspectives. Ann Chir Plast Esthet 2008;53:226-7. [Crossref] [PubMed]
- Noguchi M, Yokoi-Noguchi M, Ohno Y, et al. Oncoplastic breast conserving surgery: Volume replacement vs. volume displacement. Eur J Surg Oncol 2016;42:926-34. [Crossref] [PubMed]
- Almasad JK, Salah B. Breast reconstruction by local flaps after conserving surgery for breast cancer: an added asset to oncoplastic techniques. Breast J 2008;14:340-4. [Crossref] [PubMed]
- Hernanz F, Regaño S, Redondo-Figuero C, et al. Oncoplastic breast-conserving surgery: analysis of quadrantectomy and immediate reconstruction with latissimus dorsi flap. World J Surg 2007;31:1934-40. [Crossref] [PubMed]
- Ramakant P, Mishra A, Chand G. Oncoplastic breast-conserving surgery: analysis of quadrantectomy and immediate reconstruction with latissimus dorsi flap. World J Surg 2008;32:1569-author reply 1570. [Crossref] [PubMed]
- Hamdi M, Salgarello M, Barone-Adesi L, et al. Use of the thoracodorsal artery perforator (TDAP) flap with implant in breast reconstruction. Ann Plast Surg 2008;61:143-6. [Crossref] [PubMed]
- Clough KB, van la Parra RFD, Thygesen HH, et al. Long-term Results After Oncoplastic Surgery for Breast Cancer: A 10-year Follow-up. Ann Surg 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Veiga DF, Veiga-Filho J, Ribeiro LM, et al. Quality-of-life and self-esteem outcomes after oncoplastic breast-conserving surgery. Plast Reconstr Surg 2010;125:811-7. [Crossref] [PubMed]
- De La Cruz L, Blankenship SA, Chatterjee A, et al. Outcomes After Oncoplastic Breast-Conserving Surgery in Breast Cancer Patients: A Systematic Literature Review. Ann Surg Oncol 2016;23:3247-58. [Crossref] [PubMed]