
Risks of second primary malignancies among Chinese cancer survivors at a single center during 2002–2016
Introduction
During the last century, continuous developments in surgical procedures, radiotherapy, chemotherapy agents, and combination treatments have enabled people to live longer after a diagnosis of cancer. Between 2005 and 2015, the global number of cancer cases increased by 33%, while the number of deaths decreased for many cancers, such as Hodgkin lymphoma, esophageal cancer, stomach cancer, and chronic myeloid leukemia (1). As these improved outcomes have increased the population of cancer survivors, an increasing number of people have begun to develop other diseases, including second primary malignancies (SPMs). Second-order or higher-order malignancies now account for approximately 16% of incident cancer, based on data from the National Malignancy Institute’s Surveillance, Epidemiology, and End Results Program (2). Furthermore, cancer survivors may be especially susceptible to developing SPMs because of their unique factors, including genetic syndromes, common etiological exposures, and the late effects of chemotherapy and radiotherapy (3). Thus, given the longer duration of cancer survivorship and the substantial increase in the population of survivors who are at risk of developing SPMs, the incidence and mortality from SPMs are expected to increase.
There is ample literature regarding the risk of SPMs among specific survivor groups, such as patients with breast cancer (4) and adult leukemia (5). However, to the best of our knowledge, little is known regarding the risks of developing SPMs across the spectrum of cancer survivors who are diagnosed as having hematological malignancies, as well as the risks of hematological SPMs among patients with other malignances. As screening practices have been widely adopted for several common hematological malignancies [e.g., adult leukemia, myelodysplastic syndrome (MDS), and lymphoma], a better understanding of SPM epidemiology could help achieve better long-term outcomes for cancer survivors, who may not be covered by screening recommendations that are aimed at the broader population. Therefore, the present study aimed to examine the risks of developing SPMs among survivors of the most common hematological malignancies, as well as the risk of developing hematological SPMs among patients with other common malignances. A clearer understanding of these risks may facilitate the design of appropriate long-term surveillance strategies.
Methods
Patients
This retrospective study evaluated all patients who were treated for hematological malignancies between January 2002 and December 2016 at the Department of Hematology, Tongji Hospital, Tongji Medical College. Among the eligible patients, we identified 67 consecutive patients (≥18 years old) with a confirmed diagnosis of SPMs. The study’s retrospective protocol was approved by the ethics review board of Tongji Hospital, Tongji Medical College. All patients provided informed consent for the general collection and analysis of their data, and the study’s protocol complied with the tenets of the Declaration of Helsinki.
Definitions
The patients’ diagnoses were reviewed and reclassified according to the World Health Organization’s 2016 classification. Patients with acute myeloid leukemia (AML) were diagnosed and classified according to the French-American-British Classification, and their prognoses were analyzed according to the 2016 Revised International Prognostic Scoring System (Table 1). The exact dates of diagnosis and bone marrow examination were documented. The Warren and Gates criteria (6), with the National Cancer Institute modification (7), were used to define SPMs as a metachronous malignancy that developed ≥6 months after the first primary malignancy. We subsequently excluded 30 patients in whom the SPM was diagnosed within 6 months after the first malignancy, and 2 patients in whom the latency period was unknown, in order to prevent misclassification of metastatic primary malignancies as SPMs. Individuals with >1 pathological diagnosis of malignancy were identified and categorized as the SPM group. The latency interval was defined as the period between the diagnoses of the first malignancy and the SPM.
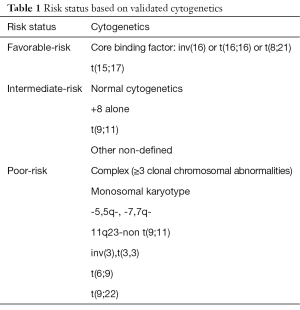
Full table
Clinical data
Clinical and treatment-related data were collected from the patients’ medical records. The 3-year survival distribution of SPMs in the study population was calculated using death as a competing event. Chemotherapy agents were classified according to the mechanism of action: alkylating agents, topoisomerase 2 inhibitors, anti-metabolites, and anti-tubulin agents (8). The study population was stratified according to sex, age, first primary malignancy, and SPM, the associations of these variables with the cumulative incidence of SPMs were determined.
Statistical evaluation
Statistical analyses of the patients’ clinical characteristics and SPMs were performed for the 67 patients who developed SPMs. The statistical analyses were performed using SPSS software (version 22; IBM Corp., Armonk, NY, USA), and the best discriminator threshold was detected using the minimal P value approach, with P values <0.05 being considered statistically significant. All tests were two-sided. Categorical variables were reported as number and frequency, and the incidence of SPMs was assumed to follow a Poisson distribution. Inter-group comparisons were performed using a non-parametric approach with the Mann-Whitney test. Comparisons of the categorical variables’ distributions were performed using the chi-square test. Survival analyses were performed using the Kaplan-Meier method and log-rank test.
Results
SPMs among cancer survivors
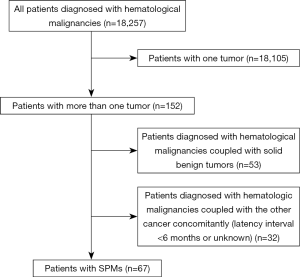
Between January 2002 and December 2016, 18,257 patients diagnosed with hematological malignancies were treated at our center. Of these, 152 patients (0.83%) had hematological malignancies with other tumors, including 67 patients (44.1%) with SPMs, 32 patients (21.1%) with other concomitant cancer (latency interval of <6 months), and 53 patients (34.9%) with concurrent benign tumors (mainly uterine fibroids) (Figure 1). Among the 67 patients with SPMs, 5 patients had a history of solid benign tumors before the first primary malignancy, 8 patients had multiple primary malignancies, and 28 patients (41.8%) had hematological malignancies as their first malignancy.
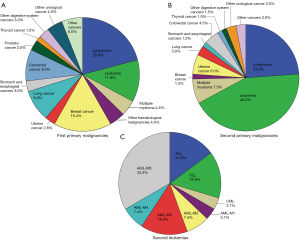
The clinical characteristics of all patients are shown in Table 2. SPMs were most common among survivors of lymphoma (mainly the diffuse, large B-cell type), which was followed by breast cancer, leukemia, colorectal cancer, and lung cancer (P<0.05) (Figure 2A). The most common SPM was leukemia (43.3% of patients), followed by lymphoma (23.9%), multiple myeloma (7.5%), uterine cancer (6.0%), and colorectal cancer (4.5%) (P<0.05) (Figure 2B). Among survivors of lymphoma, 28.6% of the SPMs were another type of lymphoma. Among survivors of breast cancer, leukemia was the most common SPM (81.8%). The most common SPMs among survivors of leukemia were lymphoma, colorectal cancer, and uterine cancer. Among patients with a second leukemia, AML-M5 was the most common French-American-British type (33.3%), followed by chronic lymphocytic leukemia, acute lymphocytic leukemia and AML-M3 (P<0.05) (Figure 2C).
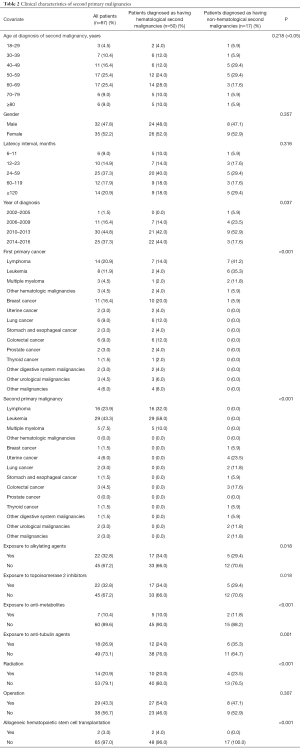
Full table
Risks among cancer survivors
The median age at the SPM diagnosis was 56.7 years (range, 18–96 years), and most patients with SPMs were 40–69 years old (67.2%) (Figure 3A). Of the 67 patients, 32 men (47.8%), although no significant sex-related differences were observed (P>0.05). There was no significant age-related difference between hematological and non-hematological malignancies (P>0.05). The diagnoses of SPMs increased during recent years (Figure 3B). The mean latency period for SPMs was 77.6 months, and the median time was 36 months, with 37.3% of the patients diagnosed with their SPMs at 24–59 months after the first diagnosis. Compared to the latency period between the first and SPMs, the latency period between the second and third malignancies was shorter (mean: 32.3 months, median: 33 months, range, 5–60 months), although the difference was not statistically significant (P>0.05). Compared to patients with first hematological malignancies, patients with non-hematological first malignancies had a longer latency period, and a similar result was observed for patients with non-hematological second malignancies (Figure 3C). However, these differences were not statistically significant (P>0.05).
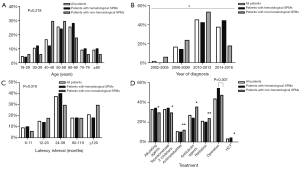
Fourteen patients (20.9%) had received radiotherapy, 35 patients (52.2%) had undergone surgery, 29 patients (43.3%) had received chemotherapy, and 2 patients (3%) had undergone allogeneic hematopoietic stem cell transplantation (HSCT). Twenty-two patients (32.8%) had received alkylating agents, 22 patients (32.8%) had received topoisomerase 2 inhibitors, 7 patients (10.4%) had received anti-metabolites, and 18 patients (26.9%) had received anti-tubulin agents. Patients with hematological SPMs had received alkylating agents and topoisomerase 2 inhibitors (34%) or undergone allogeneic HSCT (4%). These rates were significantly higher than the rates for patients with non-hematological SPMs (P<0.05). Compared to patients with hematological SPMs, patients with non-hematological SPMs had higher rates of exposure to anti-metabolites (11.8%), anti-tubulin agents (35.3%), and radiotherapy (23.5%) (P<0.05) (Figure 3D).
Cytogenetic characteristics of SPMs
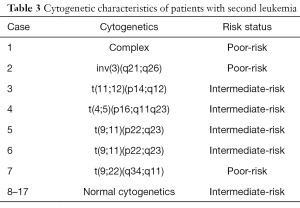
Among the 29 patients with a second leukemia, 2 patients (6.9%) had rearrangements of the MLL (11q23) gene. Seventeen patients with a second leukemia were subjected to karyotype analysis, which revealed that 14 patients (82.4%) had intermediate cytogenetics and 3 patients (17.6%) had poor cytogenetics (Table 3).
Full table
Survival analysis
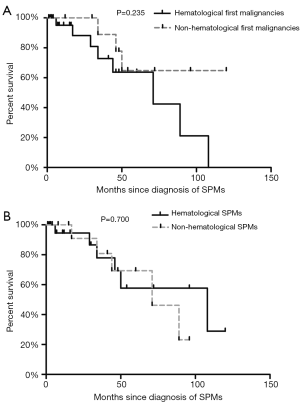
Although data regarding patients with SPMs were available up until 2016, we only analyzed the 3-year survival rate among 43 patients who were diagnosed as having SPMs before 2014. Figure 4 shows that the median follow-up was 29 months (range, 0–120 months). Longer survival was observed for patients with non-hematological first (Figure 4A) and second malignancies (Figure 4B) (vs. hematological first and second malignancies), although these differences were not statistically significant (P=0.235 and P=0.700).
Discussion
In this large population-based study at a single center, we identified 67 patients with SPMs, all of whom were diagnosed with at least one hematological malignancy. We further evaluated the risk factors for developing SPMs, and found that SPMs were more common among survivors with hematological malignancies than those with non-hematological malignancies. Patients with lymphoma had the highest risk of SPMs, and leukemia was the most common SPM. However, we did not detect any significant differences in the risks of SPMs according to age, sex, and the latency interval. To the best of our knowledge, this is the first large-scale study to systematically evaluate the characteristics of Chinese patients at a single center who were diagnosed with hematological malignancies and who experienced SPMs.
An American study indicated that the most common SPM was lung cancer, and patients with bladder cancer had the highest risk of being diagnosed as having SPMs (8). There are several possible explanations for these discrepancies. First, we evaluated SPMs in patients diagnosed with at least one hematological malignancy at a single center, while the previous study evaluated SPMs in the general population of cancer survivors in United States (3). Thus, our SPM rate may have been underestimated. Second, there may have been bias given that these SPMs could represent misclassified metastases from the primary tumor. However, we believe that this risk was minimized by our exclusion of patients who had their second malignancy diagnosed within 6 months after the first malignancy. Third, the present study evaluated a group of Chinese patients, whereas the previous study evaluated American patients and 85% of those patients were white people (3). Thus, racial and regional differences may explain the discrepancies between our findings and the previous findings. Last, we performed a retrospective study and could not control for various factors (e.g., smoking, diet, radiotherapy, surgery, HSCT, or chemotherapy), which could have influenced the incidence of SPMs. Therefore, unmeasured covariates may have affected both the primary cancer and development of SPM.
The present study’s results revealed that the risk of hematological SPMs (vs. non-hematological SPMs) was increased among patients who had been treated using alkylating agents, topoisomerase 2 inhibitors, or allogeneic HSCT for the first cancer. In contrast, the risk of non-hematological SPMs was increased among patients who had been treated using anti-metabolites, anti-tubulin agents, or radiotherapy for the first cancer. To the best of our knowledge, our study is the first one to observe this difference.
Chemotherapy using anthracyclines for breast cancer or topoisomerase inhibitors for leukemia can increase the risk of SPMs, especially AML. This is because the treatment kills cancer cells through DNA damage, although hidden latent damage to the DNA of normal cells can eventually cause new cancers (9). For example, a study of 234 patients receiving fludarabine-based, cyclophosphamide-based, and rituximab-based first-line regimens revealed that their risk of second cancers was 2.38× higher than the expected risk in the general population (10). Long-term radiation exposure is also associated with carcinogenesis, despite being an important part of multimodality therapy for many malignancies, and 14 of our patients with SPMs (20.9%) had received radiotherapy. Kamran et al. (11) also reported that radiotherapy appeared to increase the risk of SPMs in primary hematological, breast, gynecological, and pediatric malignancies. Radivoyevitch et al. (5) reported that patients who underwent radiotherapy for prostate cancer had an increased risk of AML and MDS that peaked at 1.5–2.5 years. This increased risk is also associated with age, hormone levels, chemotherapy use, environmental factors, genetic predisposition, infection, and immunosuppression, although it is difficult to define the dose-response relationship for developing SPMs after radiotherapy (11).
HSCT is a double-edged sword that can cause SPMs and/or mortality after successful treatment of the primary disease. For example, the overall risk of secondary MDS/AML is higher among patients who undergo allogeneic HSCT (vs. other treatments), and the estimated risk of SPMs after allogeneic HSCT is 3.3× higher that the risk in the general population (12,13). In the present study, 2 patients had undergone allogeneic HSCT for their first malignancy. Local factors (e.g., chronic skin inflammation and radiation damage) and profound immunosuppression (e.g., chronic graft-versus-host disease and immunosuppressive drug use) may have influenced the development of SPMs after bone marrow transplantation (14). Alam et al. (15) have also suggested that unrelated donors are a significant risk factor for both greater non-relapse mortality and decreased overall survival.
Only some patients who undergo chemotherapy, radiotherapy, or HSCT develop certain SPMs; this suggests that they may be genetically predisposed to primary and secondary malignancies. Genetic variation in pathways that mediate cellular responses to DNA damage can affect the risk of developing therapy-related AML, presumably by influencing the likelihood that hematopoietic cells survive with leukemogenic mutations. Ellis et al. (16) reported that two common functional p53-pathway variants (MDM2 SNP309 and the TP53 codon 72 polymorphism) interact to modulate responses to genotoxic therapy and affect the risk of therapy-related AML. Moreover, mutations in genes that drive hereditary breast cancer syndromes (e.g., BRCA2), rare mutations in five genes (CDH1, BMPR1A, STK11, PRSS1, and PMS2), and mitochondrial dysfunction (influenced by gene expression in CD34+ stem cells) can reduce the ability to neutralize reactive oxygen species that are generated through chemotherapy and radiotherapy, and subsequently lead to cancer-causing mutations (17,18). Two patients in the present study had rearrangements of the MLL (11q23) gene, which plays important roles in the regulation of homeotic gene expression and embryonic development (19). Douet-Guilbert et al. (20) analyzed 65 patients with secondary acute lymphoblastic leukemia and observed an association with 11q23/MLL rearrangement. Shima et al. (21) also suggested that MLL is crucial in the initiation of NUP98-HOXA9 leukemia. Translocations of the MLL gene also produce fusion proteins, such as MLL-AF4, which are associated with a poor prognosis in patients with leukemia (22). Moreover, genetic alterations of MLL are involved in bladder cancer relapse (23). Thus, we speculate that MLL may play a crucial role in the development of SPMs.
Hematological malignancy was the main risk factor in the present study. However, the difference between hematological and non-hematological malignancies did not reach statistical significance, which is likely related to the relatively short follow-up period and limited number of cases available for the survival analysis. Nevertheless, the difference between these two groups was noticeable. The findings of the present study also have various implications for cancer survivors, as the incidence of SPMs has been increasing during recent years. Therefore, lifelong cancer screening is recommended for all cancer survivors. Furthermore, screening and preventative strategies should incorporate the patient’s specific risk profile, which includes their age, sex, lifestyle, genetic predisposition, immunosuppression, pre-treatment exposures, and post-treatment complications.
Conclusions
Approximately 0.37% of patients diagnosed with hematological malignancies had SPMs, and the highest rate was observed among survivors with lymphoma. The most common SPM was leukemia (43.3% of all patients with SPMs). Hematological SPMs were associated with previous treatment using alkylating agents, topoisomerase 2 inhibitors, and HSCT, whereas non-hematological SPMs were associated with previous treatment anti-metabolites, anti-tubulin agents, and radiotherapy. However, there were no significant differences in the risks of SPMs according to age, sex, and the latency interval. Therefore, the growing number of Chinese cancer survivors and the high risk of SPMs in the present study suggest that it will be prudent to develop effective detection and treatment strategies for this population.
Acknowledgments
Funding: This work was supported by the Natural Science Foundation of China (grant no. 81570110).
Footnote
Conflict of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.02.12). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study’s retrospective protocol was approved by the ethics review board of Tongji Hospital, Tongji Medical College (grant no. TJ-IRB20170709). All patients provided informed consent for the general collection and analysis of their data.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Global Burden of Disease Cancer Collaboration. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 2017;3:524-48. [Crossref] [PubMed]
- Travis LB. The epidemiology of second primary cancers. Cancer Epidemiol Biomarkers Prev 2006;15:2020-6. [Crossref] [PubMed]
- Donin N, Filson C, Drakaki A, et al. Risk of second primary malignancies among cancer survivors in the United States, 1992 through 2008. Cancer 2016;122:3075-86. [Crossref] [PubMed]
- Kaplan HG, Malmgren JA, Li CI, et al. Age related risk of myelodysplastic syndrome and acute myeloid leukemia among breast cancer survivors. Breast Cancer Res Treat 2013;142:629-36. [Crossref] [PubMed]
- Radivoyevitch T, Sachs RK, Gale RP, et al. Defining AML and MDS second cancer risk dynamics after diagnoses of first cancers treated or not with radiation. Leukemia 2016;30:285-94. [Crossref] [PubMed]
- Warren S. Multiple primary malignant tumors. A survey of the literature and a statistical study. Gastroenterology 1932;93:779.
- Supramaniam R. New malignancies among cancer survivors: SEER cancer registries, 1973–2000. Journal of Epidemiology & Community Health 2006;62:375-6. [Crossref]
- Smith SM, Le Beau MM, Huo D, et al. Clinical-cytogenetic associations in 306 patients with therapy-related myelodysplasia and myeloid leukemia: the University of Chicago series. Blood 2003;102:43-52. [Crossref] [PubMed]
- Brower V. Tracking chemotherapy's effects on secondary cancers. J Natl Cancer Inst 2013;105:1421-2. [Crossref] [PubMed]
- Benjamini O, Jain P, Trinh L, et al. Second cancers in patients with chronic lymphocytic leukemia who received frontline fludarabine, cyclophosphamide and rituximab therapy: distribution and clinical outcomes. Leuk Lymphoma 2015;56:1643-50. [Crossref] [PubMed]
- Kamran SC, Berrington de Gonzalez A, Ng A, et al. Therapeutic radiation and the potential risk of second malignancies. Cancer 2016;122:1809-21. [Crossref] [PubMed]
- Forrest DL, Nevill TJ, Naiman SC, et al. Second malignancy following high-dose therapy and autologous stem cell transplantation: incidence and risk factor analysis. Bone Marrow Transplant 2003;32:915-23. [Crossref] [PubMed]
- Vaxman I, Ram R, Gafter-Gvili A, et al. Secondary malignancies following high dose therapy and autologous hematopoietic cell transplantation-systematic review and meta-analysis. Bone Marrow Transplant 2015;50:706-14. [Crossref] [PubMed]
- Lishner M, Lang R. Secondary cancers after bone marrow transplantation. N Engl J Med 1990;322:853. [Crossref] [PubMed]
- Alam N, Atenafu EG, Kuruvilla J, et al. Outcomes of patients with therapy-related AML/myelodysplastic syndrome (t-AML/MDS) following hematopoietic cell transplantation. Bone Marrow Transplant 2015;50:1180-6. [Crossref] [PubMed]
- Ellis NA, Huo D, Yildiz O, et al. MDM2 SNP309 and TP53 Arg72Pro interact to alter therapy-related acute myeloid leukemia susceptibility. Blood 2008;112:741-9. [Crossref] [PubMed]
- Li X, Kang J, Pan Q, et al. Genetic analysis in a patient with nine primary malignant neoplasms: a rare case of Li-Fraumeni syndrome. Oncol Rep 2016;35:1519-28. [Crossref] [PubMed]
- Cano KE, Li L, Bhatia S, et al. NMR-based metabolomic analysis of the molecular pathogenesis of therapy-related myelodysplasia/acute myeloid leukemia. J Proteome Res 2011;10:2873-81. [Crossref] [PubMed]
- Zhang X, Novera W, Zhang Y, et al. MLL5 (KMT2E): structure, function, and clinical relevance. Cell Mol Life Sci 2017;74:2333-44. [Crossref] [PubMed]
- Douet-Guilbert N, Eveillard JR, Meyer C, et al. MLL partner genes in secondary acute lymphoblastic leukemia: report of a new partner PRRC1 and review of the literature. Leuk Res 2014;38:1316-9. [Crossref] [PubMed]
- Shima Y, Yumoto M, Katsumoto T, et al. MLL is essential for NUP98-HOXA9-induced leukemia. Leukemia 2017;31:2200-10. [Crossref] [PubMed]
- Kerry J, Godfrey L, Repapi E, et al. MLL-AF4 Spreading Identifies Binding Sites that Are Distinct from Super-Enhancers and that Govern Sensitivity to DOT1L Inhibition in Leukemia. Cell Rep 2017;18:482-95. [Crossref] [PubMed]
- Wu S, Yang Z, Ye R, et al. Novel variants in MLL confer to bladder cancer recurrence identified by whole-exome sequencing. Oncotarget 2016;7:2629-45. [PubMed]


