
Prognostic values of preoperative NLR and PLR in patients with laryngeal squamous cell carcinoma
Introduction
Laryngeal squamous cell carcinoma (LSCC) is a common head and neck cancer that remains as a major reason of morbidity and mortality. Estimates of 26,400 new cases of laryngeal cancer and 14,500 laryngeal cancer-related deaths in each year in China were reported (1), and the estimates of 13,430 and 3,620, respectively, in the United States (2). Treatment choices for LSCC include operation, radiotherapy, and chemotherapy either used alone or in combination (3-7). In spite of the progresses in diagnostic and treatment methods, the clinical prognosis of LSCC has not been significantly improved in the past 30 years. Finding effective biomarkers to identify patients who are prone to relapse or metastases and administering personalized therapy may improve the clinical outcomes.
Recently, many studies have revealed on the association between inflammation and malignancies. Inflammation and activation of the immune system have antitumor activity. They also play roles in carcinogenesis and the progression of cancers (8). The neutrophil-lymphocyte ratio (NLR) and the platelet-lymphocyte ratio (PLR) are simple and effective markers of inflammation and immunity. NLR was studied in few cardiovascular diseases and different cancers and was found to be related with recurrence, tumor aggressiveness, and poor prognosis (9-12). Platelets can activate tumor growth by accumulating angiogenesis, improving microvascular permeableness, and facilitating the extravasation of cancer cells (13). PLR was also validated to have prognostic value in several types of cancer such as esophageal squamous cell cancer, gastric cancer and lung cancer (14-16). The prognostic values of NLR and PLR in LSCC has also been reported, but none of the studies focused on the prognostic value of the combination of NLR and PLR in LSCC. Therefore, we conducted the present study to assess the prognostic values of preoperative NLR and PLR in LSCC patients.
Methods
Patient population
The clinical data of patients with LSCC who underwent radical resection at the Guangdong General Hospital & Guangdong Academy of Medical Sciences between January 2006 and August 2011 and the Sun Yat-sen University Cancer Center between October 2007 and December 2009 were collected. Staging of LSCC was based on the American Joint Committee on Cancer (AJCC) TNM staging system (7th edition, 2010) (17). The diagnosis of LSCC was validated by postoperative pathological analysis. The selective standards for the patients were as follows: (I) older than 18 years; (II) complete clinical, laboratory, imaging, and follow-up data; (III) no chronic infectious diseases; (IV) no hematological disorders or treatment that can result in an elevated NLR and PLR, for example, administration of hematopoietic drugs such as granulocyte-colony stimulating factor (G-CSF) within one month before operation; (V) no autoimmune disease or preoperative usage of steroids; (VI) no preoperative treatments such as chemotherapy and radiotherapy. Routine blood tests were implemented on the day before operation. NLR and PLR were calculated as the absolute neutrophil or platelet counts, respectively, divided by the absolute lymphocyte counts. Patients were followed up every 3 months until 30th September 2014 in the Guangdong General Hospital and 31st May 2015 in the Sun Yat-sen University Cancer Center. The overall survival (OS) was calculated from the time of operation to the date of death or the last visit. The study was approved by the Research Ethics Committee of Guangdong General Hospital & Guangdong Academy of Medical Sciences and Sun Yat-sen University Cancer Center [No. GDREC2013245H (R1)]. All participants signed informed consent.
Statistical analysis
All statistical analyses were accomplished with Statistical Product and Service Solutions (SPSS) version 19.0 software (Chicago, IL, USA). The sensitivity and specificity of the NLR and PLR for predicting OS using by receiver operating characteristic (ROC) curve analysis. The Youden index was estimated to select the best cut-off value of NLR and PLR. The relationship between clinicopathologic features and dichotomized NLR and PLR was compared by the chi-square test. Survival analysis were calculated by the Kaplan-Meier method then compared using the log-rank test. The multiple Cox proportional hazards model (a forward stepwise procedure) was performed to identify independent prognostic factors based on variables selected on univariate analysis (factors analyzed by univariate analysis with P<0.05). P<0.05 was considered significant.
Results
Demographic data
A total of 336 LSCC patients underwent radical resection. Among them, 9 patients received preoperative treatments, 11 patients showed clinical evidence or hematological diseases and other infectious conditions, and 26 patients without the clinical data were excluded. Finally, 290 patients 282 men and 8 women with a median age of 62 (interquartile range, 41–86) years were selected in this study. Of the 290 patients, 214 (73.8%) had smoking history (18), and 73 (25.2%) had drinking history (19) 100 (34.5%) underwent total laryngectomy, 111 (38.3%) underwent partial laryngectomy, and 79 (27.2%) underwent CO2 laser surgery;184 (63.4%) were in stage T1–T2 and 106 (36.6%) in stage T3–T4; 226 (77.9%) had tumor located in the glottic region, 52 (17.9%) in the supraglottic region and 12 (4.2%) in the subglottic region; 52 (17.9%) had lymph node metastasis.
Determination of cut-off value of NLR and PLR
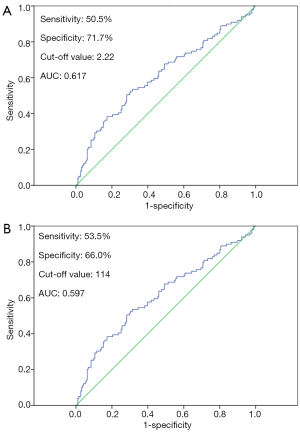
The results of ROC curve analysis showed that the optimal cut-off value of preoperative NLR was 2.22, with the maximum joint sensitivity of 50.5% and specificity of 71.7% [area under curve (AUC): 0.617; 95% confidence internal (CI): 0.546–0.687; P=0.001]. Then, patients were divided into the NLR
Relationships between NLR, PLR, and clinicopathologic characteristics
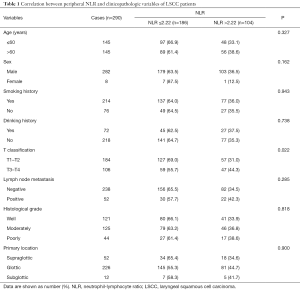
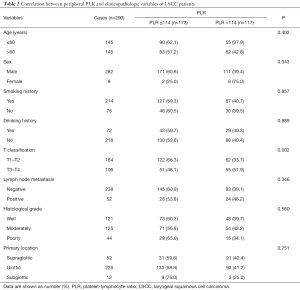
The relationships between NLR, PLR, and clinicopathologic features of patients are showed in Tables 1 and 2. An increase in NLR was significantly related with more advanced T stage (P=0.022). An increase in PLR was also significantly related with more advanced T classification (P=0.002). Furthermore, more man patients were found in the low PLR group (P=0.043). No noteworthy difference in age, smoking history, drinking history, lymph node metastasis, histological grade, and primary location were discovered between the low and high NLR groups, or between the low and high PLR groups.
Full table
Full table
Prognostic features of LSCC patients
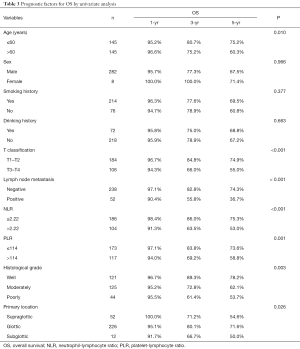
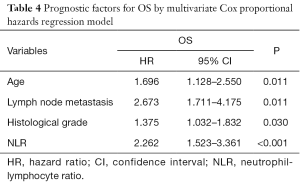
To classify the risk factors affecting postoperative OS, we evaluated NLR, PLR, and clinicopathologic factors in univariate and multivariate analyses. Univariate analysis identified age, T stage, lymph node metastasis, NLR, PLR, histological grade, and primary tumor location as significant prognostic features of OS (Table 3). Multivariate analysis identified four parameters, including age, lymph node metastasis, histological grade, and NLR as independent prognostic factors of OS for LSCC patients (all P<0.05) (Table 4).
Full table
Full table
OS according to preoperative NLR and PLR
The patients were followed up for a median of 64 months (range, 3–102 months). During follow-up period, 191 patients were alive, and 99 died. The 1-, 3-, and 5-year OS rates in the NLR ≤2.22 group were significantly higher than those in the NLR >2.22 group (98.4%, 86.0% and 75.3% vs. 91.3%, 63.5% and 53.0%, P<0.001) (Figure 2A). Similarly, the 1-, 3-, and 5-year OS rates in the PLR ≤114 group were distinctly higher than those in the PLR >114 group (97.1%, 83.8% and 73.6% vs. 94.0%, 69.2% and 58.8%, respectively, P=0.001) (Figure 2B).
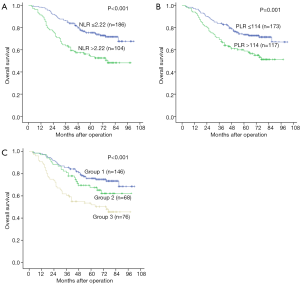
Prognostic value of the combination of NLR-PLR in LSCC patients
To analyze the prognostic value of NLR combined with PLR in LSCC after surgery, patients were divided into three groups: patients with normal NLR and normal PLR were allocated to group 1 (n=146), with elevated NLR or elevated PLR were allocated to group 2 (n=68), and with elevated NLR and elevated PLR were allocated to group 3 (n=76). The 1-, 3-, and 5-year OS rates of the group 1 were markedly higher than those in the groups 2 and 3 (97.9%, 85.6%, and 75.8% vs. 97.1%, 80.9%, and 67.5% and 90.8%, 60.5%, and 51.9%, respectively, P<0.001) (Figure 2C).
Discussion
To improve the prognosis of LSCC patients, great effort was made to seek for effective prognostic markers of LSCC. NLR and PLR are simple and effective markers of inflammation and immunization. They reveal the inflammatory and immune condition of human body, increases the amounts of circulating neutrophils and promotes neutrophils secreting angiogenesis-regulating growth factors, chemokines and proteases (e.g., vascular endothelial growth factor (VEGF), interleukin-8 (IL-8), intercellular adhesion molecule 1 and matrix metalloproteinase (MMP)) which may facilitate cancer progression and metastasis (20-23). NLR and PLR have also been presented to be valued prognostic indicators in several cancers, but all of these hadn’t investigated the relationship between NLR and PLR in LSCC, especially the prognostic value of their combination. The objective of this study was to develop a simple risk assessment model based on NLR and PLR to improve the prophecy of survival in LSCC patients, which were an innovation of our research. In previous studies, the cut-off value of NLR and PLR were different, including head and neck cancer (24). The cut-off value has been set empirically or basing on the median value.
In the present study, to avoid the empirical bias, the cut-off values of NLR and PLR were selected according to ROC curve analysis. The best cut-off values of NLR and PLR were 2.22 and 114, respectively. We further analyzed the relationships between NLR, PLR and clinicopathological characteristics of LSCC patients, and found that both elevated NLR and elevated PLR were correlated with advanced T stage. The results indicated that NLR or PLR could not only reflect the tumor burden but also stimulate tumor progress by affecting the tumor metabolism and microenvironment. We discovered several prognostic factors of OS for LSCC patients, such as age, T stage, lymph node metastasis, NLR, PLR, histological grade, and primary tumor location by using univariate analysis. After multivariate analysis, we finally found that the independent prognostic factors of OS were age, lymph node metastasis, histological grade, and NLR.
We discovered that both high NLR and high PLR were associated with short OS of LSCC patients, which were in compliance with the results from previous studies (25-27). As we know that a combination of multiple markers might produce more information for predicting clinical outcome. We combined NLR with PLR to predict the prognosis of LSCC patients. We classified the patients into three groups and developed a simple risk assessment model according to the levels of NLR and PLR. Our results displayed that patients with both normal NLR and normal PLR had the best prognosis, whereas patients with both elevated NLR and elevated PLR had the worst prognosis. This risk model confirmed our hypothesis that the accuracy in predicting prognosis of LSCC can be boosted through the combination of NLR and PLR.
There are some limitations in the present study. First, it was a retrospective study with a relatively small size. And a well-designed, prospective study with a large number of patients is needed to confirm our findings. Second, postoperative radiotherapy or chemotherapy is an important prognostic factor. However, we only investigated the preoperative clinicopathological factors, and the post-operative treatments were not considered in the present study. Third, although we found that elevated NLR or PLR predicted poor prognosis and that the accuracy in prognosis prediction can be enhanced by the combination of them and these patients might benefit from postoperative adjuvant treatments, we were not capable of verifying the assumption, which we desire to validate in future clinical trials.
Conclusions
This study demonstrates that preoperative elevated NLR and elevated PLR are prognostic factors for LSCC patients. Preoperative NLR and PLR provide us an effective support to discern patients at high hazard of death. Moreover, their combination can promote the prognostic accuracy for survival of LSCC patients. These results may advise us to take the therapy proposal thinking about not only TNM stage but also these prognostic markers associated blood routine. However, the exact mechanisms and function of NLR and PLR on LSCC should been elucidated. In the future, the simple preoperative prognosis assessment could be utilized to select patients for adapted treatment.
Acknowledgments
Funding: None.
Footnote
Conflict of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.03.03). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Research Ethics Committee of Guangdong General Hospital & Guangdong Academy of Medical Sciences and Sun Yat-sen University Cancer Center [No. GDREC2013245H (R1)]. All participants signed informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Tai P, Yu E, Shiels R, et al. Long-term survival rates of laryngeal cancer patients treated by radiation and surgery, radiation alone, and surgery alone: studied by lognormal and Kaplan–Meier survival methods. BMC Cancer 2005;5:13. [Crossref] [PubMed]
- Ghaffar S, Akhtar S, Ikram M, et al. Comparison of different treatment modalities in advanced laryngeal hypopharyngeal squamous cell carcinoma. J Coll Physicians Surg Pak 2010;20:171-4. [PubMed]
- Varghese BT, Sebastian P, Mathew A. Treatment outcome in patients undergoing surgery for carcinoma larynx and hypopharynx: a follow-up study. Acta Otolaryngol 2009;129:1480-5. [Crossref] [PubMed]
- Mahler V, Boysen M, Brøndbo K. Radiotherapy or CO(2) laser surgery as treatment of T(1a) glottic carcinoma? Eur Arch Otorhinolaryngol 2010;267:743-50. [Crossref] [PubMed]
- Bajaj Y, Shayah A, Sethi N, et al. Clinical outcomes of total laryngectomy for laryngeal carcinoma. Kathmandu Univ Med J (KUMJ) 2009;7:258-62. [PubMed]
- Dmitrieva OS, Shilovskiy IP, Khaitov MR, et al. Interleukins 1 and 6 as main mediators of inflammation. Biochemistry (Mosc) 2016;81:80-90. [Crossref] [PubMed]
- Gazi E, Bayram B, Gazi S, et al. Prognostic value of the neutrophil–lymphocyte ratio in patients with ST-elevated acute myocardial infarction. Clin Appl Thromb Hemost 2015;21:155-9. [Crossref] [PubMed]
- Fu SJ, Shen SL, Li SQ, et al. Prognostic value of preoperative peripheral neutrophil-to-lymphocyte ratio in patients with HBV-associated hepatocellular carcinoma after radical hepatectomy. Med Oncol 2013;30:721. [Crossref] [PubMed]
- Ozturk K, Akyildiz NS, Uslu M, et al. The effect of preoperative neutrophil, platelet and lymphocyte counts on local recurrence and survival in early-stage tongue cancer. Eur Arch Otorhinolaryngol 2016;273:4425-9. [Crossref] [PubMed]
- Chen S, Guo J, Feng C, et al. The preoperative platelet–lymphocyte ratio versus neutrophil-lymphocyte ratio: which is better as a prognostic factor in oral squamous cell carcinoma? Ther Adv Med Oncol 2016;8:160-7. [Crossref] [PubMed]
- Sabrkhany S, Griffioen AW, Oude Egbrink MG. The role of blood platelets in tumor angiogenesis. Biochim Biophys Acta 2011;1815:189-96. [PubMed]
- Xie X, Luo KJ, Hu Y, et al. Prognostic value of preoperative platelet-lymphocyte and neutrophil- lymphocyte ratio in patients undergoing surgery for esophageal squamous cell cancer. Dis Esophagus 2016;29:79-85. [Crossref] [PubMed]
- Jiang N, Deng JY, Liu Y, et al. The role of preoperative neutrophil-lymphocyte and platelet- lymphocyte ratio in patients after radical resection for gastric cancer. Biomarkers 2014;19:444-51. [Crossref] [PubMed]
- Cannon NA, Meyer J, Iyengar P, et al. Neutrophil-lymphocyte and platelet-lymphocyte ratios as prognostic factors after stereotactic radiation therapy for early-stage non-small-cell lung cancer. J Thorac Oncol 2015;10:280-5. [Crossref] [PubMed]
- Edge SB. Cancer staging manual, 7th ed. New York: Springer; 2010.
- Zhang SY, Lu ZM, Luo XN, et al. Retrospective analysis of prognostic factors in 205 patients with laryngeal squamous cell carcinoma who underwent surgical treatment. PLoS One 2013;8:e60157 [Crossref] [PubMed]
- Imai R, Takenaka Y, Yasui T, et al. Prognostic significance of serum squamous cell carcinoma antigen in patients with head and neck cancer. Acta Otolaryngol 2015;135:295-301. [Crossref] [PubMed]
- Fondevila C, Metges JP, Fuster J, et al. p53 and VEGF expression are independent predictors of tumour recurrence and survival following curative resection of gastric cancer. Br J Cancer 2004;90:206-15. [Crossref] [PubMed]
- Schaider H, Oka M, Bogenrieder T, et al. Differential response of primary and metastatic melanomas to neutrophils attracted by IL-8. Int J Cancer 2003;103:335-43. [Crossref] [PubMed]
- Liu S, Li N, Yu X, et al. Expression of intercellular adhesion molecule 1 by hepatocellular carcinoma stem cells and circulating tumor cells. Gastroenterology 2013;144:1031-41.e10. [Crossref] [PubMed]
- Sivaramakrishnan V, Niranjali Devaraj S. Morin regulates the expression of NF-κB-p65, COX-2 and matrix metalloproteinases in diethylnitrosamine induced rat hepatocellular carcinoma. Chem Biol Interact 2009;180:353-9. [Crossref] [PubMed]
- Takenaka Y, Oya R, Kitamiura T, et al. Prognostic role of neutrophil-to-lymphocyte ratio in head and neck cancer: A meta-analysis. Head Neck 2018;40:647-55. [Crossref] [PubMed]
- Tu XP, Qiu QH, Chen LS, et al. Preoperative neutrophil-to-lymphocyte ratio is an independent prognostic marker in patients with laryngeal squamous cell carcinoma. BMC Cancer 2015;15:743. [Crossref] [PubMed]
- Kara M, Uysal S, Altinişik U, et al. The pre-treatment neutrophil-to- lymphocyte ratio, platelet-to-lymphocyte ratio, and red cell distribution width predict prognosis in patients with laryngeal carcinoma. Eur Arch Otorhinolaryngol 2017;274:535-42. [Crossref] [PubMed]
- Chen WZ, Yu ST, Xie R, et al. Preoperative albumin/globulin ratio has predictive value for patients with laryngeal squamous cell carcinoma. Oncotarget 2017;8:48240-7. [PubMed]


