
Basic characteristics and therapy regimens for colorectal squamous cell carcinoma
Introduction
Colorectal cancer (CRC) is one of the leading causes of cancer-related death in males and females worldwide (1). The incidence and mortality of CRC both rank in the top five of all cancers in China (2). Adenocarcinoma makes up more than 90% of CRC (3,4). Surgery is commonly used as the primary therapeutic regimen, and the chemoradiation would also be closed in some cases with the consideration of staging, localization, and the patient's situation (5,6). There are several rare types of CRC tumors such as malignant carcinoid (1.5%), malignant lymphoma (0.6%), neuroendocrine carcinoma (0.3%), squamous cell carcinoma (SCC, 0.3%), and others (7). SCC is a small subset of CRC, accounting for less than 1% of CRC incidence (7,8). The incidence of rectal SCC (93.4%) is higher than colonic SCC (5.9%) (7). The most common site of SCC in the lower gastrointestinal tract is anal canal. Unlike SCC of the anal canal, colorectal SCC is much less reported. Most studies regarding colorectal SCC are limited to case reports, and the etiology is still unclear (9). There are varying hypothesizes about the etiology of colorectal SCC, such as differentiation of a pluripotent stem cell or the squamous metaplasia resulting from external irritation (10,11). Chronic inflammation or viral infection may also promote the development of colorectal SCC (9,12). However, the definite etiology of colorectal SCC remains to be discerned. Surgery was once the standard treatment for colorectal SCC, with tumor location and depth of invasion considered in operation selection (5,13). However, there is no distinct recommended treatment in the National Comprehensive Cancer Network guideline (3). In recent years, the treatment for SCC of the rectum and of the colon differs in that non-metastatic colonic SCC is only treated by surgery, while rectal SCC has the option of chemoradiotherapy with or without surgery or with surgery alone (14). Besides, there are few investigations about the treatment of the non-metastatic colonic SCC, which lacks the unified treatment standards.
In our study, we aim to compare the characteristics of colorectal SCC with different histological subtypes and different primary tumor sites using the Surveillance, Epidemiology, and End Results Program (SEER) database. We also analyze the prognosis between patients who received different therapeutic regimens using both of the SEER and SEER-Medicare linked database to help determine the optimal treatment regimen for colorectal SCC.
Methods
Data for SEER database set
This study was a retrospective investigation. Data were obtained from the SEER Program and SEER-Medicare linked databases. The study met the requirements of the SEER data use agreement. The SEER database is a population-based cancer registry accounting for approximately 28% of the US population among widespread regions, containing information including demographics, tumor characteristics, survival information, and cause of death for cancer patients.
Data for SEER-Medicare database set
For the SEER-Medicare set, this investigation was performed following the requirements of SEER-Medicare data use agreement, and approval was obtained from the First Hospital of China Medical University Institutional Review Board. The SEER-Medicare database is the primary health insurer which accounts for about 97% of the US population ≥66 years old (15).
Patients and variables for SEER database set
The World Health Organization (WHO) International Classification of Diseases for Oncology, 3rd edition (ICD-O-3) was used to determine histological tumor types. Patients from the SEER database included in this study were diagnosed between 1988 and 2014. These patients were diagnosed with five histological types of CRC (site codes: 18.0, 18.2–18.9, 19.9, 20.9), including adenocarcinoma [8140–8147], mucinous adenocarcinomas [8480, 8481], signet ring cell carcinoma [8490], SCC [8070–8078], and adenosquamous carcinoma [8560, 8562]. The exclusion criteria for patients were: (I) a diagnosis of CRC or any other cancers within 1 year after the first admission; (II) had previous cancer diagnosis; (III) tumor-node-metastasis (TNM) stage was 0 or missing; (IV) incomplete histological type information; (V) survival time was 0 or missing.
Basic patient characteristics such as age, sex, histological grade, pathological stage, race, marital status, and diagnosis year were compared between patients with different histological types of CRC or different primary sites of SCC using data from the SEER database. Pathological stage was confirmed via the seventh edition of the Union for International Cancer Control (UICC) TNM staging system.
Patients and variables for SEER-Medicare database set
Patients from the SEER-Medicare database was aged 66 years or older and with a primary diagnosis of CRC between 1992 and 2009. All of these patients were diagnosed with colorectal (site codes: 18.0, 18.2–18.9, 19.9, 20.9) SCC [8070–8078]. The exclusion criteria for patients were: (I) a diagnosis of CRC or any other cancers within 1 year after the first admission; (II) had previous cancer diagnosis; (III) lacked full coverage of Medicare Parts A and B from 12 months before through to 12 months after diagnosis if not dead, or were enrolled in a health maintenance organization (HMO); (IV) TNM stage was 0 or missing; (V) incomplete histological type information; (VI) survival time was 0 or missing. The detailed drug codes used in our study were based on National Drug Code and Health Care Financing Administration Common Procedure Coding System, which has been reported previously (16).
Basic patient characteristics from the SEER-Medicare database such as age, sex, histological grade, pathological stage, race, marital status, and diagnosis year were also analyzed similar to SEER dataset. Besides, residence, median household income, level of education, Hierarchical Condition Category (HCC), performing operation or not, chemotherapy, and radiotherapy were also analyzed using the SEER-Medicare database set. Centers for Medicare and Medicaid Services Hierarchical Condition Categories were used for risk adjustment, which is based on the outpatient and inpatient diagnoses from the 12 months before CRC diagnosis. The resulting score can be regarded as a prediction of patient’s “future health care need” with the influence caused by the average Medicare beneficiary (HCC =1.0) (17). Pathological stage was confirmed via the seventh edition of the UICC TNM staging system.
Statistical analysis
All analyses were performed using SPSS 20.0 (Somers, NY, USA) software and R3.3.1 (Vienna, Austria). Comparisons of patient demographics and characteristics between different histological types or tumor sites were performed using the χ2 test. To analyze the primary outcome, overall survival (OS), the Kaplan-Meier method was used. The log-rank test was used to compare survival curves. To control the influence of patient characteristics, the Cox proportional hazards model was used for the multivariate analysis. Variables which were significantly associated with survival in the univariate analysis were accepted as covariates in the Cox proportional hazards model. P values <0.05 were defined as statistically significant.
Results
Patients and tumor characteristics for SEER database set
A total of 365,202 patients were included in our study. From the SEER database, 365,098 CRC patients were included with five histological types: adenocarcinoma (n=316,835), mucinous adenocarcinoma (n=43,257), signet ring cell carcinoma (n=4,375), SCC (n=377), and adenosquamous carcinoma (n=254). We found that colorectal SCC is more common in females (64.7%) and it tends to have higher pT category and TNM stage. The most common site of colorectal SCC is the rectum (80.6%). Demographic characteristics for the five histological types are shown in Table 1.
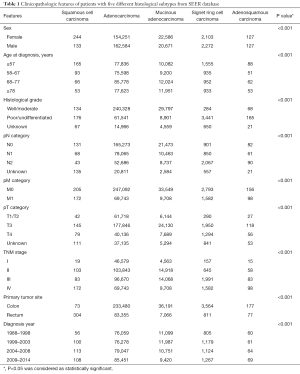
Full table
Patients and tumor characteristics for SEER-Medicare database set
We included a total of 104 colorectal SCC patients from the SEER-Medicare database to investigate therapy outcomes. A higher incidence of the colorectal SCC was also seen in the females (63.5%). Besides, higher TNM stage and incidence of the rectum SCC were also found. Basic patient characteristics are shown in Table 2.
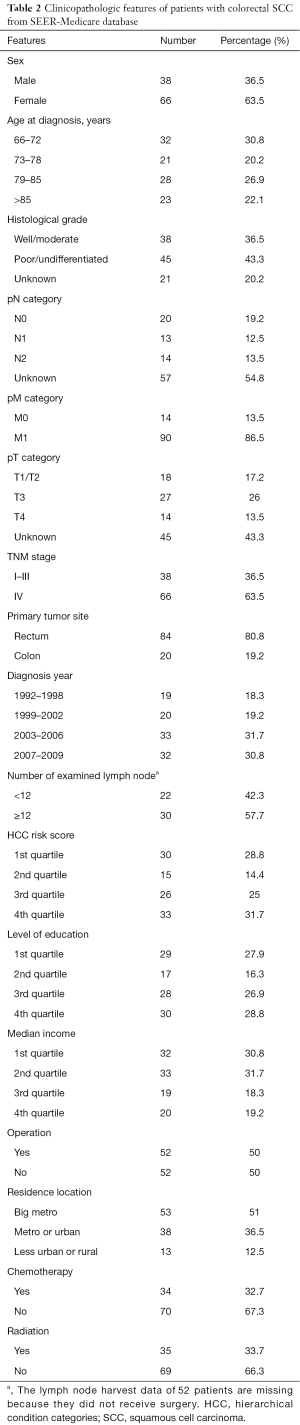
Full table
Survival analysis for colorectal SCC patients from the SEER database
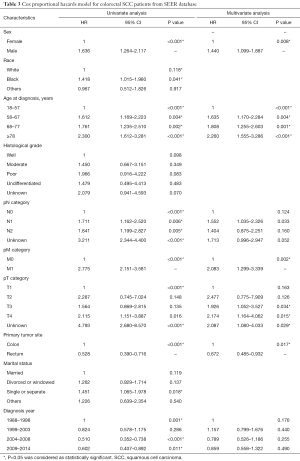
We first analyzed 5-year survival rate for the five histological types of CRC for patients from the SEER database set using the Kaplan-Meier method. The results indicated that 5-year survival rate was significantly lower for SCC patients (35.0%, 95% CI: 29.9–40.1%) than adenocarcinoma (54.6%, 95% CI: 54.5–54.8%, P<0.001) and mucinous adenocarcinoma (51.4%, 95% CI: 50.9–51.9%, P<0.001). Furthermore, the 5-year survival rate of SCC was significantly higher than signet ring cell carcinoma (27.9%, 95% CI: 26.5–29.3%, P=0.041), and had no significant difference compared with adenosquamous carcinoma (33.7%, 95% CI: 27.7–39.9%, P=0.775, Figure 1A). We also compared the 5-year survival rate between five histological types of CRC stratified by TNM stage. The prognosis of the colorectal SCC patients was significantly worse than adenocarcinoma both in stage I–III patients (50.8% vs. 64.5%, P<0.001, Figure 1B) and stage IV patients (16.0% vs. 19.5%, P=0.002, Figure 1C), which was also significantly lower than mucinous adenocarcinomas both in stage I–III (50.8% vs. 61.0%, P=0.011, Figure 1B) and stage IV patients (16.0% vs. 17.8%, P=0.019, Figure 1C). Besides, the prognosis of the colorectal SCC patients was significantly better than signet ring cell carcinoma both in stage I–III patients (50.8% vs. 40.2%, P=0.005, Figure 1B) and stage IV patients (16.0% vs. 6.3%, P=0.015, Figure 1C). The Cox proportional hazards model was then used for the multivariate survival analysis for colorectal SCC. We found that sex and primary tumor site were independent prognosis factors for colorectal SCC, and females and patients with rectal SCC had better prognosis. Age and pM category were both independent negative prognosis factors for colorectal SCC. Details are shown in Table 3.
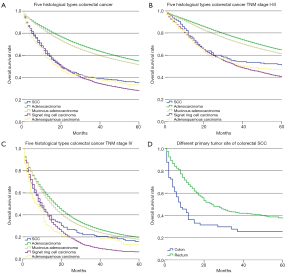
Full table
To analyze the influence of primary tumor site on prognosis, we filtered data from the SEER database, which was stratified by primary tumor site including colon and rectum. The Kaplan-Meier method was used to compare the different 5-year survival rate between these two groups. We found a significantly increasing 5-year survival rate for patients with colonic SCC (25.5%) and rectal SCC (37.3%, P<0.001, Figure 1D).
Treatment regiments analysis of colorectal SCC
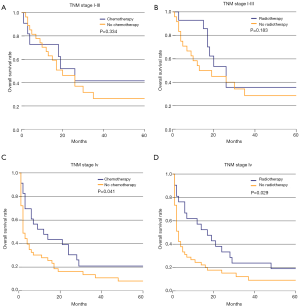
Firstly, we divided the colorectal SCC patients from the SEER database into two groups according to TNM stage. In the stage I–III group, patients received surgery with chemoradiotherapy shared a little bit higher 5-year survival rate (56.1%) than the chemoradiotherapy alone (49.9%, P=0.767), and better than surgery along (38.7%, P=0.001) or no treatment (40.0%, P=0.365, Figure 2A). In stage IV patients, we also found that patients received surgery with chemoradiotherapy have the best prognosis (31.0%) among no treatment (7.8% vs. 31.0%, P<0.001), surgery alone (11.8% vs. 31.0%, P=0.004), and chemoradiotherapy alone (14.5% vs. 31.0%, P=0.184, Figure 2B). Due to the widely reported about the treatment paradigm shift of rectal SCC towards definitive chemoradiotherapy in recent years, we also analyzed the prognosis of rectal SCC patients receiving different treatment. In the stage I–III group, we found that patients received chemoradiotherapy alone shared a similar 5-year survival rate with the patients received surgery with chemoradiotherapy (54.9% vs. 56.5%, P=0.565), which is better than surgery alone (54.9% vs. 39.7%, P=0.151) and no treatment (54.9% vs. 40.0%, P=0.365, Figure 2C), but failed to reach statistical significance. In stage IV group, the rectal SCC patients receiving chemoradiotherapy alone had a significantly higher 5-year survival rate then no treatment (15.6% vs. 5.6%, P<0.001) and surgery alone (15.6% vs. 12.5%, P=0.018). However, the patients receiving chemoradiotherapy alone had a significantly lower 5-year survival rate than patients having surgery with chemoradiotherapy (15.6% vs. 39.4%, P=0.043, Figure 2D).

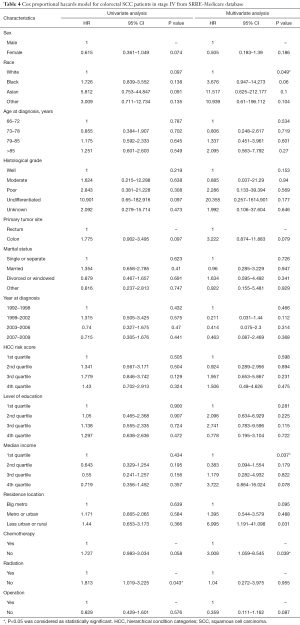
We also divided the colorectal SCC patients from the SEER-Medicare database into two groups according to TNM stage. In the stage I–III group, patients with no operation were excluded due to the small number. According to the Kaplan-Meier survival analysis, chemotherapy (41.6% vs. 26.4%, P=0.334) and radiotherapy (35.6% vs. 28.6%, P=0.183) could improve survival rate to some extent in stage I–III patients who underwent an operation, but failed to reach statistical significance (Figure 3A,B). In stage IV patients, we found that chemotherapy (20.9% vs. 8.1%, P=0.041) and radiotherapy (19.0% vs. 9.1%, P=0.029) could both obviously improve 5-year survival rate (Figure 3C,D). However, only chemotherapy was found to be an independent prognostic indicator for colorectal SCC patients from the results of the multivariate survival analysis (HR 3.008, 95% CI: 1.059–8.545, P=0.039). Operation performance (HR 0.359, 95% CI: 0.111–1.162, P=0.087) and radiation (HR 1.04, 95% CI: 0.272–3.975, P=0.955) did not show significant prognostic improvements from the results of the multivariate survival analysis. Besides, race and income levels were both independent prognostic indicators for colorectal SCC patients (Table 4).
Full table
Discussion
The diagnosis of primary colorectal SCC must meet the following requirements: the primary tumor site should be colonic or rectal, the lesions should not be involved in any squamous-lined fistula, and rectal SCC should be excluded for tumors arising from the anal squamous epithelium (11). It is widely accepted that the incidence of colorectal SCC is much lower than adenocarcinoma. For this reason, most studies on colorectal SCC are case reports (7,9,18). Details such as etiology, characteristics, and therapy guideline are still undefined for colorectal SCC.
In our study, we analyzed the characteristics of colorectal SCC using population-based data from the SEER and SEER-Medicare databases. We found that females made up a larger portion of colorectal SCC patients than males, and sex was an independent prognostic factor for colorectal SCC, similar to previous investigations (7,19). Most colorectal SCC is diagnosed with moderate or poor differentiation (75.9%), and rectal SCC (80.6%) accounts for a large portion. From the survival analysis, we found that the prognosis of colorectal SCC is much worse than for adenocarcinoma (35.0% vs. 54.6%, P<0.001) or mucinous adenocarcinoma (35.0% vs. 51.4%, P<0.001), and better than ring cell carcinoma (35.0% vs. 27.9%, P=0.041), but no significant prognostic difference existed between colorectal SCC and adenosquamous carcinoma. Masoomi et al. similarly found that SCC had a higher mortality than adenocarcinoma (20). There are some factors may affect the prognosis of colorectal SCC. First of all, the rectal SCC may have a higher propensity for frequently locally invasive and metastatic dissemination when compared with adenocarcinoma, which may probably cause by a delayed diagnosis (21,22). Thus, the locally invasion and metastasis would contribute to the poor prognosis of colorectal SCC. Besides, the recent studies have given a global paradigm shift from surgery towards definitive chemoradiotherapy to improve the rectal SCC patients’ prognosis. However, some investigators reported that rectal SCC may be less radiosensitive than its histologic counterparts, which may lead to a worse prognosis (3).
We also analyzed survival rates between different primary tumor sites, finding a significantly increasing 5-year survival rate among colonic and rectal SCC (25.5% vs. 37.3%, P<0.001). The five-year survival rate for the squamous cell rectal cancer among the literature have varied from 32–86%, which limited by small sample size (3,7,23-25). In our study, the 5-year survival rate was lower than many of studies, which may be caused by the following reasons. Firstly, the ratio of the stage IV patients in our study was higher (45.6%). The 5-year survival rate of stage I–III rectal SCC patients was 52.3%, which was much higher than stage IV patients (18.2%). Kang et al. also reported that the 5-year survival rate of the stage IV patients was 20.8%, which was lower than other stages (7). Therefore, the heavy preponderance of stage IV patients may also lead to the poor prognosis of rectal SCC. Secondly, we included the patients diagnosed in a long-time span [1988–2014] from the SEER database, and the 5-year survival rate are different among different periods (19.6% for 1988–1998 group, 39% for 1999–2003 group, 45.3% for 2004–2008 group, 35.4% for 2009–2014 group). The treatment efficiency of the rectal SCC was unsatisfied in the early years, which has been improved in recent years. Kulaylat et al. has reported that the 5-year survival rate of the rectal SCC patients was 66.8% who was diagnosed between 2006–2012 (3). Besides, Kang et al. also reported the 5-year survival rate of the colorectal SCC patients diagnosed between 1991 and 2000 was 48.9%, which is much lower than Kulaylat’s result (7). Thus, the different period has the different treatment regimens, which may lead to the different prognosis of rectal SCC patients.
No optimal therapy regimens have been established for colorectal SCC due to its low incidence. For rectal SCC, surgery was once the standard treatment, with tumor location and depth of invasion considered in operation selection (4,13). With medical advancements, chemoradiotherapy has gradually been accepted as the standard treatment for anal SCC since their introduction by Nigro in the 1970s (26). These findings strongly influenced the treatment of rectal SCC. Thus, many investigations have evaluated its therapeutic efficacy, showing that chemoradiotherapy could lead to higher local control rate, longer survival time, and a high rate of organ preservation for rectal SCC (24,27). However, some studies still reported that surgery was useful to help improve survival rates for rectal SCC (23,28). Besides, the current literature of colorectal SCC consists primarily of case reports, case series, and lacks the large population-based study. Thus, the treatment for colorectal SCC is still controversial. From the SEER database, we found that colorectal SCC patients received surgery with chemoradiotherapy treatment had a higher 5-year survival rate than surgery alone and chemoradiotherapy alone. For stage I–III rectal SCC patients, patients received chemoradiotherapy alone shared a similar 5-year survival rate than surgery with chemoradiotherapy treatment (54.9% vs. 56.5%, P=0.565), which is better than surgery alone (39.7%, P=0.151), but failed to reach statistical significance. In stage IV group, the rectal SCC patients receiving chemoradiotherapy alone had a significantly higher 5-year survival rate then no treatment and surgery alone. In SEER-Medicare database, we found that most stage I–III patients underwent surgery. In these patients, chemotherapy (41.6% vs. 26.4%) and radiotherapy (35.6% vs. 28.6%) improved the 5-year survival rate, though not with statistical significance, possibly due to the limited patient numbers. For stage IV patients, chemotherapy was an independent prognosis factor (HR 3.008, 95% CI: 1.059–8.545, P=0.039). In conclusion, the chemoradiotherapy may improve the prognosis of the colorectal SCC patients.
Although we have analyzed the characteristics of colorectal SCC from different angles, there are still many limitations. First, our study is retrospectively, and the SEER database only represents less than a third of the US population. The results may be biased in terms of socioeconomic and other factors. Second, the small number of colorectal SCC patients in some groups prevents us from performing a deeper investigation delineating the optimal treatment for colorectal SCC. Third, the results of the SEER database identifies the 18–57 years old age group as the highest incidence group, and the patients in SEER database were diagnosed between 1988 and 2013. However, the SEER-Medicare database only contains patients older than 65 years and diagnosed between 1992 and 2009. These differences would introduce significant bias when attempting to assess the effect of treatment regimen. Fourth, there is a heavy preponderance of stage IV patients in our study, which indicates that many of the operation may have been undertaken with a palliative rather than curative intent, which had a great impact on patients’ outcomes and the results. Fifth, stage I–III patients with no operation were excluded due to the small number in the SEER-Medicare database, which is unusual given the paradigm shift in the treatment of rectal SCC towards definitive chemoradiotherapy in recent years. However, many patients in our study have an unclear TNM stage because they did not receive operation, and have been excluded according to the exclusion criteria of our study. Besides, the included patients were diagnosed between 1992 and 2009 in the SEER-Medicare database. The treatment paradigm for SCC of the rectum primarily involves surgery in that period. Thus, most of the stage I–III patients received an operation in our study. In conclusion, further multi-center, large population-based analyses should be performed to clarify guidelines for the treatment of colorectal SCC.
Conclusions
Our study shows that the 5-year survival rate for colorectal SCC is much lower than adenocarcinoma or mucinous adenocarcinoma, and similar to signet ring cell carcinoma and adenosquamous carcinoma. Patients with rectal SCC had a better prognosis compared with colon SCC.
Acknowledgments
We acknowledge Joseph Burclaff from the Washington University School of Medicine for providing language help and writing assistance.
Funding: This work was supported by the Clinical Capability Construction Project for Liaoning Provincial Hospitals (LNCCC-A01-2014), the Key Laboratory Programme of Education Department of Liaoning Province (LZ2015076), and the National Key R&D Program of China (MOST-2017YFC0908300, MOST-2017YFC0908305), Key Laboratory Grant of Education Department of Liaoning Province (LS201602).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.03.04). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Review by the institutional review board was not required for this study as the SEER database is publicly available without individually identifiable private information. Informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Kulaylat AS, Hollenbeak CS, Stewart DB Sr. Squamous Cancers of the Rectum Demonstrate Poorer Survival and Increased Need for Salvage Surgery Compared With Squamous Cancers of the Anus. Dis Colon Rectum 2017;60:922-7. [Crossref] [PubMed]
- Wang JF, Wang ZX, Xu XX, et al. Primary rectal squamous cell carcinoma treated with surgery and radiotherapy. World J Gastroenterol 2014;20:4106-9. [Crossref] [PubMed]
- Labianca R, Nordlinger B, Beretta GD, et al. Early colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24:vi64-72. [Crossref] [PubMed]
- Glimelius B, Tiret E, Cervantes A, et al. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24:vi81-8. [Crossref] [PubMed]
- Kang H, O’Connell JB, Leonardi MJ, et al. Rare tumors of the colon and rectum: a national review. Int J Colorectal Dis 2007;22:183-9. [Crossref] [PubMed]
- DiSario JA, Burt RW, Kendrick ML, et al. Colorectal cancers of rare histologic types compared with adenocarcinomas. Dis Colon Rectum 1994;37:1277-80. [Crossref] [PubMed]
- Ozuner G, Aytac E, Gorgun E, et al. Colorectal squamous cell carcinoma: a rare tumor with poor prognosis. Int J Colorectal Dis 2015;30:127-30. [Crossref] [PubMed]
- Frizelle FA, Hobday KS, Batts KP, et al. Adenosquamous and squamous carcinoma of the colon and upper rectum: a clinical and histopathologic study. Dis Colon Rectum 2001;44:341-6. [Crossref] [PubMed]
- Williams GT, Blackshaw AJ, Morson BC. Squamous carcinoma of the colorectum and its genesis. J Pathol 1979;129:139-47. [Crossref] [PubMed]
- Wiener MF, Polayes SH, Yidi R. Squamous carcinoma with schistosomiasis of the colon. Am J Gastroenterol 1962;37:48-54. [PubMed]
- Guerra GR, Kong CH, Warrier SK, et al. Primary squamous cell carcinoma of the rectum: An update and implications for treatment. World J Gastrointest Surg 2016;8:252-65. [Crossref] [PubMed]
- Delhorme JB, Waissi W, Romain B, et al. Management of rectal squamous cell carcinoma. Clin Res Hepatol Gastroenterol 2017;41:e71-3. [Crossref] [PubMed]
- Potosky AL, Riley GF, Lubitz JD, et al. Potential for cancer related health services research using a linked Medicare-tumor registry database. Med Care 1993;31:732-48. [Crossref] [PubMed]
- Gao P, Song YX, Sun JX, et al. Which is the best postoperative chemotherapy regimen in patients with rectal cancer after neoadjuvant therapy? BMC Cancer 2014;14:888. [Crossref] [PubMed]
- Ash AS, Ellis RP, Pope GC, et al. Using diagnoses to describe populations and predict costs. Health Care Financ Rev 2000;21:7-28. [PubMed]
- Juturi JV, Francis B, Koontz PW, et al. Squamous-cell carcinoma of the colon responsive to combination chemotherapy: report of two cases and review of the literature. Dis Colon Rectum 1999;42:102-9. [Crossref] [PubMed]
- Yeh J, Hastings J, Rao A, et al. Squamous cell carcinoma of the rectum: a single institution experience. Tech Coloproctol 2012;16:349-54. [Crossref] [PubMed]
- Masoomi H, Ziogas A, Lin BS, et al. Population-based evaluation of adenosquamous carcinoma of the colon and rectum. Dis Colon Rectum 2012;55:509-14. [Crossref] [PubMed]
- Gelas T, Peyrat P, Francois Y, et al. Primary squamous-cell carcinoma of the rectum: report of six cases and review of the literature. Dis Colon Rectum 2002;45:1535-40. [Crossref] [PubMed]
- Wang ML, Heriot A, Leong T, et al. Chemoradiotherapy in the management of primary squamous-cell carcinoma of the rectum. Colorectal Dis 2011;13:296-301. [Crossref] [PubMed]
- Steinemann DC, Muller PC, Billeter AT, et al. Surgery is essential in squamous cell cancer of the rectum. Langenbecks Arch Surg 2017;402:1055-62. [Crossref] [PubMed]
- Loganadane G, Servagi-Vernat S, Schernberg A, et al. Chemoradiation in rectal squamous cell carcinoma: Bi-institutional case series. Eur J Cancer 2016;58:83-9. [Crossref] [PubMed]
- Dyson T, Draganov PV. Squamous cell cancer of the rectum. World J Gastroenterol 2009;15:4380-6. [Crossref] [PubMed]
- Nigro ND, Vaitkevicius VK, Buroker T, et al. Combined therapy for cancer of the anal canal. Dis Colon Rectum 1981;24:73-5. [Crossref] [PubMed]
- Jeong BG, Kim DY, Kim SY. Concurrent chemoradiotherapy for squamous cell carcinoma of the rectum. Hepatogastroenterology 2013;60:512-6. [PubMed]
- Arora N, Gupta A, Zhu H, et al. Race- and Sex-Based Disparities in the Therapy and Outcomes of Squamous Cell Carcinoma of the Anus. J Natl Compr Canc Netw 2017;15:998-1004. [Crossref] [PubMed]


