
Pharmacologically relevant concentrations of berberine transiently stimulate dihydrotestosterone-inducible androgen receptor-mediated luciferase activity in human prostate cancer cells
Introduction
Berberine (Figure 1A) is a quaternary isoquinoline alkaloid which is used as a remedy for the treatment of diarrhea and gastroenteritis due to its anti-motility and antimicrobial activities (1). It is sold as dietary supplement with content of Berberine usually ranging from 400 to 1,000 mg per capsule (e.g., Swanson, Dr. Whitaker, aSquared Nutrition). Among many described biological effects, the antineoplastic activity attracts the attention. It was described to be effective against pancreatic cancer cells, breast cancer cells or prostate cancer cells (2-4). One of the first studies demonstrated the decline in the viability of androgen-dependent as well independent cells upon Berberine treatment as a result of inhibition of cyclins expression and activation of caspases (4). Following studies confirmed this observation and extended the knowledge about other molecular targets involved in the anti-growth activity of Berberine, like p53 (5) or c-Jun N-terminal kinase (JNK) (6). Among molecular targets of Berberine, androgen receptor (AR) was also demonstrated (7). By employing reporter gene assay, it was demonstrated that Berberine inhibited dose-dependently ligand-independent AR-dependent luciferase activity after 6 h for concentrations from 25–100 µM in 22Rv1 cells and ligand-dependent AR-dependent expression of KLK3 mRNA (prostate specific antigen; PSA) in LNCaP cells after 24 h (7). Moreover, downregulation of PSA and androgen receptor protein were demonstrated as well.
The common thing for all these studies is relatively high concentration of Berberine. Even though Berberine inhibited LNCaP xenografts in nude mice after daily i.p. injection of 5 mg/kg, the plasma concentrations in mice were not established. Moreover, concentrations which cause more than 20% decline in viability of cells in vitro are difficult to be connected with AR action especially when apoptotic events are triggered at these concentrations. Thus, the AR-dependence in human subjects is in question.
Pharmacokinetics of Berberine was determined in rats, where oral administration of 90 mg/kg led to CMAX equal to 29 ng/mL (corresponds to approx. 86 nM) with TMAX 1 h, but then it dropped down (8). Studies in human volunteers demonstrated variable pharmacokinetic profiles. In one study, single oral dose of 300 mg of Berberine resulted in Cmax =394.7±155.4 µg/L (approx. 1.17±0.46 µM) with Tmax = 2.37±0.04 h in healthy human male volunteers (9). In another study, 500 mg orally administered Berberine chloride led to Cmax of 1.4 nM in healthy volunteers (10). However, plasma levels of Berberine were higher approx. 3-times in hypercholesterolemic subjects after daily chronic administration of Berberine chloride at 15 mg/kg of body weight. Thus, with similar initial dose, concentration in plasma may vary in 3 orders of magnitude among human volunteers. In another study, daily administration of 1.2 g for 2 weeks resulted in average plasma concentration 0.19 mg/L (approx. 565 nM) (11). If we accept as the upper limit the maximal concentration from the study by Li et al. (9), then the maximal reachable systemic concentration of Berberine does not exceed 1 µM after single oral administration. Thus, higher concentrations may not be of clinical relevance. Since there was described that some compounds targeting steroid receptors may display a hormetic effect (12), it is worth to know if submicromolar concentrations of Berberine may display such a behavior or provide regular monotonic dose-response. To this purpose, we decided to investigate the effect of Berberine in low submicromolar concentrations, which more reflect real in vivo situations, on androgen receptor activity and proliferation in prostate cancer cell lines.
Methods
Compounds and reagents
Dimethyl sulfoxide (DMSO), dihydrotestosterone (DHT), Berberine, proliferation assay kit (5-Bromo-2'-deoxy-uridine Labeling and Detection Kit III), Charcoal-stripped fetal bovine serum (FBS; F6765) were purchased from Sigma-Aldrich (Prague, Czech Republic). Oligonucleotide primers used in RT-PCR reactions were synthesized by Generi Biotech (Hradec Kralove, Czech Republic). Annexin V binding buffer and Annexin V-CF488A conjugate were from Biotium (Fremont, USA). LightCycler 480 Probes Master was from Roche Diagnostic Corporation (Intes Bohemia, Czech Republic). Microtiter plates for 3D cell culture were obtained from 300Microns (Karlsruhe, Germany). All other chemicals were of the highest quality commercially available.
Cell cultures
Human Caucasian prostate carcinoma (LNCaP; No. 89110211) and human prostate carcinoma epithelial cell line (22Rv1; No. 05092802) were purchased from Public Health England. Both cell lines and stably transfected cell line AIZ-AR (derived from 22Rv1) were cultured in RPMI 1640 medium supplemented with 10% of charcoal-stripped fetal bovine serum, 100 U/mL streptomycin, 100 µg/mL penicillin, 2 mM L-glutamine, 1% non-essential amino acids, and 1 mM sodium pyruvate. Cells were maintained at 37 °C and 5% CO2 in a humidified incubator.
Cell viability assay (MTT)
Cell line AIZ-AR was treated with increasing concentrations of BER (0.01–50 µM) and/or DMSO (0.1%; v/v) for 24 h. Thereafter, the medium was replaced by PBS with MTT (MTT = 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) in final concentration of 0.3 mg/mL. The solution was discarded after 30–40 minutes of incubation and replaced by DMSO for dissolution of the formazan crystals. Absorbance was measured at 570 nm with Infinite M200 (TECAN, Austria). Tested concentrations causing decline in viability no greater than 20% were considered as non-toxic for furthers experiments.
Gene reporter assay
Stably transfected gene reporter cell line AIZ-AR, which was derived from 22Rv1 cells transfected with a construct containing 3 copies of androgen response regions (ARRs) followed by a single copy of androgen response element (ARE) from the promoter region of human PSA (KLK3) gene (Bartonkova, 2015). Following the plating, cells were stabilized for 16 h and then treated with BER (0.001–1,000 nM) in the absence (Agonist setting) or presence of dihydrotestosterone (DHT; 100 nM) (Antagonist setting) and/or vehicle (DMSO; 0.1% v/v) for 24 h. After the treatments, cells were lysed and luciferase activity was measured with Infinite M200 (TECAN, Austria).
Quantitative reverse transcriptase polymerase chain reaction (qRT-PCR)
The total RNA was isolated using TRI Reagent® (Molecular Research Center, USA). cDNA was synthesized from 1,000 ng of total RNA using M-MuLV Reverse Transcriptase (M0253S, New England BioLabs) at 42 °C for 60 min in the presence of random hexamers (S1230, New England BioLabs). qRT-PCR was carried out on Light Cycler 480 II apparatus (Roche Diagnostic Corporation, Prague, Czech Republic). The levels of KLK3 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNAs were determined using primers and Universal Probes Library (UPL; Roche Diagnostic Corporation, Prague, Czech Republic) probes as follows: KLK3 (PSA)-UPL 44, forward: GTGCTTGTGGCCTCTCGT, reverse: CAGCAAGATCACGCTTTTGT; GAPDH-UPL 60, forward: CTCTGCTCCTCCTGTTCGAC, reverse: ACGACCAAATCCGTTGACTC. The following program was used for monitoring the expression of all genes: an activation step at 95 °C for 10 min was followed by 45 cycles of PCR (denaturation at 95 °C for 10 s; annealing with elongation at 60 °C for 30 s). The measurements were performed in triplicates. Gene expression was normalized per GAPDH as a housekeeping gene. Data were processed by the delta-delta method. Results are expressed as fold induction over DMSO-treated cells.
Proliferation assay
Proliferation assay was performed according to the manufacturer recommendations with minor modifications. Briefly, cells were seeded at the density of 10,000 cells per well into 96-well plate (for LNCaP pre-coated with poly-D-lysine; for 3D culture with microcavities each with 300 µm in diameter) and stabilized overnight. Next day, the cells were treated with dihydrotestosterone (DHT) alone or together with increasing concentrations of Berberine (0.1–1,000 nM) for 24 h. At the end of treatment, cells were washed with PBS and the solution with 5-Bromo-2'-deoxy-uridine (BrDu) was applied for 3 h. Thereafter, we followed strictly the procedure from manufacturer guide. At the end, the absorbance at 405 nm and reference 495 nm were measured with Infinite M200Pro (Tecan, Austria). The proliferation capacity was calculated as % of the ratio of absorbances A405/A495 for DHT control sample and for other samples, i.e., DHT sample was set to 100%.
Annexin V assay
Annexin V assay was performed according to the manufacturer recommendations (ChemoMetec). Briefly, 22Rv1 cells were seeded at the density of 4×105 cells per well into 12-well plate in RPMI medium supplemented with Charcoal-stripped serum. After overnight stabilization, cells were treated with dihydrotestosterone (DHT; 100 nM), Berberine (1,000 nM), combination of both and/or DMSO (0.1%, v/v) for 24 h. Thereafter, cells were harvested by trypsinization, counted and approx. 3×105 cells were resuspended in Annexin V binding buffer, to which Annexin V-CF488A and Hoechst 33342 were added. After incubation at 37 °C for 15 min, cells were spinned down twice and each time the pellet was resuspended in Annexin V binding buffer, lastly supplemented with propidium iodide and immediately analyzed by Nucleocounter NC-3000 (ChemoMetec; Denmark).
Statistical analysis
All values were subjected to Paired Student’s t-test. All statistical analyses were performed using Microsoft Excel 2010. P<0.05 was considered statistically significant.
Results
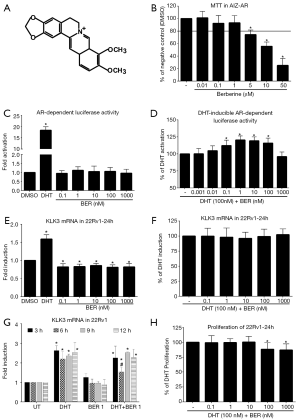
First, we determined the non-toxic concentrations of BER in recently developed AR-responsive cell line (AIZ-AR) (13) for the purpose of monitoring activity of AR. The viability significantly dropped below our personally set 80% threshold for concentration of 5 µM within 24 h (Figure 1B). Therefore, we further used concentrations no higher than 1,000 nM. In reporter gene assay, there was no activation of AR by BER next to positive control DHT (100 nM) (Figure 1C) but there was significant approx. Twenty percent increase of DHT-inducible AR-dependent luciferase activity above DHT when co-incubated with BER (Figure 1D). Based on this finding, we further focused on the expression of AR target gene, KLK3. We used 22Rv1 cells, which AIZ-AR was derived from. While the positive control DHT induced significantly KLK3 mRNA, Berberine significantly decreased the basal level of KLK3 mRNA about 20% independently of the dose (Figure 1E). However, Berberine had no effect on DHT-inducible mRNA level of KLK3 (Figure 1F). Since KLK3 mRNA may undergo different stabilization/degradation in contrast to exogenous luciferase in longer time, we monitored DHT-inducible KLK3 mRNA in shorter periods of time in the presence of Berberine (Figure 1G). We observed significantly attenuated DHT-inducible AR-dependent KLK3 mRNA in the presence of Berberine after 6 hrs. Shorter or longer duration of treatment had no effect on inducible KLK3 mRNA.
Since there was mild but significant synergism of DHT and Berberine on AR activity in reporter gene assay, we tested if this finding can be connected to proliferation of 22Rv1 cells. We observed mild but significant decline of proliferation for concentrations 100 and 1,000 nM (Figure 1H). This is in contrast to expected AR co-activation. Due to the known observations, which suggested different behavior of cells either in 2D or 3D culture model (14-16), we performed the proliferation assay in the 3D culture [technology based on the presence of microcavities (300Microns, Germany)] plates to compare 2D and 3D outputs. However, proliferation in 3D layout followed the same pattern as in the case of 2D culture (data not shown).
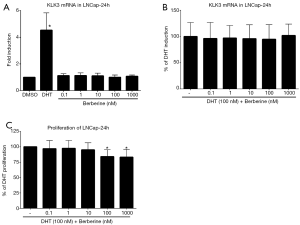
Since all experiments so far were performed in androgen-independent prostate cell line, we monitored the KLK3 expression and cellular proliferation in the presence of Berberine in androgen-dependent cell line, LNCaP. However, basal or DHT-inducible KLK3 mRNA as well as proliferation profiles were almost identical with 22Rv1 cells (Figure 2), i.e., no difference was observed.
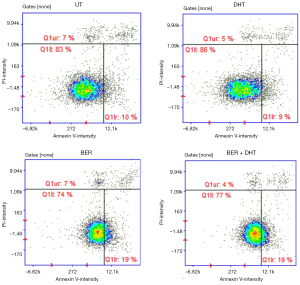
In order to find a possible reason of lower proliferation activity in the presence of Berberine, we considered a triggering of an apoptotic event. Therefore, we further focused on measuring the Annexin V intensity, a marker of apoptosis. We observed that Berberine increased Annexin V-CF488A staining next to DMSO or DHT, suggesting an early apoptotic event (Figure 3; right sector in each sub-figure). Since this effect was unaffected by DHT, it can be concluded that it does not involve androgen receptor.
Discussion
In the current paper we investigated the effect of natural alkaloid Berberine at pharmacologically relevant concentrations towards androgen receptor activity and its relation to proliferation of prostate cancer cell lines. We observed stimulation of dihydrotestosterone-inducible AR-mediated luciferase activity by Berberine in the range of concentrations 0.1–100 nM (Figure 1D). However, the presence of Berberine did not affect inducible level of AR-target gene, KLK3, in androgen-dependent (LNCaP) or independent cells (22Rv1) (Figures 1F,2B). Monitoring the proliferation revealed mild inhibition of proliferation for 100 and 1,000 nM concentrations of Berberine, which is in accordance with literature (7). Similarly, proliferation of 22Rv1 cells in 3D cell culture displayed the same but weaker pattern as in 2D culture (data not shown). Moreover, we demonstrated an initiation of apoptosis, measured as increased intensity of Annexin V staining.
Berberine is a natural compound with many biological activities, some of them beneficial for human subjects. However, the benefits are always needed to be connected with the dose/concentration. Two publications, which focused on the pharmacokinetics of Berberine in human volunteers after single oral dose administration, found controversial results as similar orally given doses (which can be obtained with commercially available dietary supplements) resulted in the different maximal plasmatic concentrations which differed approximately 1,000-times (9,10). Thus, there is a question if orally given Berberine can display such huge number of beneficial effects, which are often attributed to its use, if the dose/concentration would be in nanomolar range. Positive findings, which are often reported for Berberine using mice with xenografts as in vivo model close to cancer patients, usually administer Berberine subcutaneously, which is quite different route of exposure from oral administration and it is unlikely to be applied for cancer patients.
The study by Li et al. demonstrated that high doses of Berberine (up to 100 µM) can inhibit viability of prostate cancer cells and KLK3 induction after 6 h (7). Moreover, they demonstrated induction of apoptosis and decline in PSA as well as AR protein content. However, used concentrations were too high to have any implication on real in vivo situation. A study, which measured pharmacokinetics of 200 mg/kg orally administered Berberine in rats, described the higher amount of Berberine in some tissues next to plasma (17). However, in any case the concentrations did not exceed 1 µM and consequently any higher concentration tested may not be of clinical relevance. Nevertheless, even the use of submicromolar concentrations, we used in our study, may give some relevant answers. The anticancer effect of Berberine can happen in low concentrations as we demonstrated by proliferation assay (Figure 1H) and by Annexin V assay (Figure 3). Possibly, the real in vivo effect may be stronger in longer periods and with repeated dosing, especially for human subjects.
The main question which rises from our observation is: Does Berberine have any significant impact on prostate cancer in concentrations found in plasma? Based on our data, we might conclude that the synergistic action of nanomolar concentrations of Berberine with DHT-inducible AR-dependent activity probably represents a false positive result of our reporter assay. The interaction of AR with sole response element without the remaining distal parts as in the case of interaction with the full promoter of KLK3 gene, can certainly give different results. Moreover, an accumulation of luciferase enzyme unlike KLK3 mRNA (undergoing stabilization/degradation processes) can be one of the plausible explanations. On the other hand, luciferase activity statistically higher for combination of DHT and Berberine next to DHT alone (Figure 1D) might reflect an intracellular event, which was triggered by Berberine. Such event might be the transient activation of extracellular signal regulated kinase (ERK) via binding of Berberine to EGFR. This rapid activation was observed for environmental pollutant bisphenol A (18), which triggered expression and phosphorylation of p53 protein at ser15 residue and resulted in the cell cycle arrest. This is consistent with our data from proliferation assay and with the observation where Berberine strongly induced p53 expression in AR-positive LNCaP cells but not in AR-negative PC-3 cells at concentrations comparable with those used in our work (5). Thus, there might be an interaction of Berberine-activated kinase with AR, which we observed at AR-activity level but not at AR-target gene expression level probably due to the reasons mentioned above. Since AR was demonstrated to interact with certain type of kinases at cellular membrane and this interaction was a driving force for the development of peptides mimicking the receptor sequences responsible for these interactions (19), it can be speculated [in the context of above mentioned study (5)] that Berberine would be a suitable supportive treatment in AR-positive cancers.
In conclusion, Berberine has anti-proliferative effect on androgen-independent as well as dependent prostate cancer cells in nanomolar concentrations which links relevant plasmatic concentrations found in human subjects with its anti-cancer activity.
Acknowledgments
Funding: This work was supported by the grant from Czech Science Foundation GACR P303/12/G163.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.03.31). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The institutional ethical approval and informed consent were waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Xiao CW, Ji QA, Wei Q, et al. Antifungal activity of berberine hydrochloride and palmatine hydrochloride against Microsporum canis -induced dermatitis in rabbits and underlying mechanism. BMC Complement Altern Med 2015;15:177. [Crossref] [PubMed]
- Pinto-Garcia L, Efferth T, Torres A, et al. Berberine inhibits cell growth and mediates caspase-independent cell death in human pancreatic cancer cells. Planta Med 2010;76:1155-61. [Crossref] [PubMed]
- Kim JB, Yu JH, Ko E, et al. The alkaloid Berberine inhibits the growth of Anoikis-resistant MCF-7 and MDA-MB-231 breast cancer cell lines by inducing cell cycle arrest. Phytomedicine 2010;17:436-40. [Crossref] [PubMed]
- Mantena SK, Sharma SD, Katiyar SK. Berberine, a natural product, induces G1-phase cell cycle arrest and caspase-3-dependent apoptosis in human prostate carcinoma cells. Mol Cancer Ther 2006;5:296-308. [Crossref] [PubMed]
- Choi MS, Oh JH, Kim SM, et al. Berberine inhibits p53-dependent cell growth through induction of apoptosis of prostate cancer cells. Int J Oncol 2009;34:1221-30. [PubMed]
- Hur JM, Kim D. Berberine inhibited radioresistant effects and enhanced anti-tumor effects in the irradiated-human prostate cancer cells. Toxicol Res 2010;26:109-15. [Crossref] [PubMed]
- Li J, Cao B, Liu X, et al. Berberine suppresses androgen receptor signaling in prostate cancer. Mol Cancer Ther 2011;10:1346-56. [Crossref] [PubMed]
- Liu M, Su X, Li G, et al. Validated UPLC-MS/MS method for simultaneous determination of simvastatin, simvastatin hydroxy acid and berberine in rat plasma: Application to the drug-drug pharmacokinetic interaction study of simvastatin combined with berberine after oral administration in rats. J Chromatogr B Analyt Technol Biomed Life Sci 2015;1006:8-15. [Crossref] [PubMed]
- Li B, Zhang M, Bao L. Study on the pharmacokinetics of berberine after oral administration in human being. Journal of Harbin Medical University 1996;29:382-5.
- Spinozzi S, Colliva C, Camborata C, et al. Berberine and its metabolites: relationship between physicochemical properties and plasma levels after administration to human subjects. J Nat Prod 2014;77:766-72. [Crossref] [PubMed]
- Zeng X, Zeng X. Relationship between the clinical effects of berberine on severe congestive heart failure and its concentration in plasma studied by HPLC. Biomed Chromatogr 1999;13:442-4. [Crossref] [PubMed]
- Fagin D. Toxicology: The learning curve. Nature 2012;490:462-5. [Crossref] [PubMed]
- Bartonkova I, Novotna A, Dvorak Z. Novel stably transfected human reporter cell line AIZ-AR as a tool for an assessment of human androgen receptor transcriptional activity. PLoS One 2015;10:e0121316 [Crossref] [PubMed]
- Fischbach C, Kong HJ, Hsiong SX, et al. Cancer cell angiogenic capability is regulated by 3D culture and integrin engagement. Proc Natl Acad Sci U S A 2009;106:399-404. [Crossref] [PubMed]
- Sung KE, Su X, Berthier E, et al. Understanding the impact of 2D and 3D fibroblast cultures on in vitro breast cancer models. PLoS One 2013;8:e76373 [Crossref] [PubMed]
- Imamura Y, Mukohara T, Shimono Y, et al. Comparison of 2D- and 3D-culture models as drug-testing platforms in breast cancer. Oncol Rep 2015;33:1837-43. [Crossref] [PubMed]
- Tan XS, Ma JY, Feng R, et al. Tissue distribution of berberine and its metabolites after oral administration in rats. PLoS One 2013;8:e77969 [Crossref] [PubMed]
- Bilancio A, Bontempo P, Donato MD, et al. Bisphenol A induces cell cycle arrest in primary and prostate cancer cells through EGFR/ERK/p53 signaling pathway activation. Oncotarget 2017;8:115620-31. [Crossref] [PubMed]
- Migliaccio A, Castoria G, de Falco A, et al. Polyproline and Tat transduction peptides in the study of the rapid actions of steroid receptors. Steroids 2012;77:974-8. [Crossref] [PubMed]


