
How many pancreatic cysts are out there and how to best manage them?
The finding of an incidental asymptomatic cyst in the pancreas with routine radiographic imaging makes both patients and physicians question, “What level of ‘risk’ is acceptable for either surveillance or for an invasive intervention?” and “What price should be paid for the only approach we have to cure pancreatic cancer—early detection and adequate resection?” This topic has been the focus of several recent reviews (1-3). This issue has become clinically relevant over the last few decades since more pancreatic cysts are diagnosed with each higher quality magnetic resonance imaging (MRI) performed in an aging population (4,5). While there are international and professional societies management guidelines to help plan immediate and long term sequences of various invasive and non-invasive interventions (6-8) the evidence supporting many recommendations is weak (9), debated (10,11) and following these recommendations can be expensive (5), reflecting the incomplete knowledge currently available on their natural history and the still evolving diagnostic and interventional tools available to describe their etiology, natural course and malignant potential. High quality studies are needed to help refine and perfect future guidelines.
In this regard the study by Kromrey et al. (12) entitled, “Prospective study on the incidence, prevalence and 5-year pancreatic-related mortality of pancreatic cysts in a population-based study”, adds to the pancreatic cystic lesion knowledge base a prospective population cohort study with another answer to an apparently very simple question: how many pancreatic cysts can one anticipate finding on an MRI performed in the general asymptomatic population and do they pose a risk for development of pancreatic cancer?
The answer is unfortunately very often not straight forward. The evaluation and surveillance of high-risk pancreatic lesions and individuals in multidisciplinary pancreatic cysts programs is emerging as a comprehensively way to evaluate and risk stratify the significance of each findings for a given patient, leading in many cases to less invasive and more confident decisions and to better answers for the frequently raised question: what can one physician tell an asymptomatic patient who while having an MRI for an unrelated indication is told there is a “small cyst” in the pancreas? The involvement of a gastroenterologist, radiologist, surgeon, oncologist and geneticist is needed to better answer this and the underlying question: what is the etiology of the cyst and what is the risk of malignancy? (13).
In this study by Kromrey et al. (12), an asymptomatic population cohort had two MRIs performed 5 years apart, leading to an unexpectedly high 49.1% prevalence of pancreatic cystic lesions and new incidence of lesions of 12.9%. To put this high number in perspective of previous studies, the American Gastroenterological Association (AGA) based its recommendations (7) on an averaged prevalence of pancreatic cystic lesions of 15% in the general population and the broad range reported in various asymptomatic or high risk populations from 2.4% to 38.8% (Table 1). Factors such as the patient’s age and history of pancreatic disease increase the prevalence, number and size of pancreatic cysts, paralleling the various types of pancreatic cysts met in clinical practice, as does the diagnostic modality. The best noninvasive diagnostic modality remains the MRI (19), with endoscopic ultrasound increasing the diagnostic yield over CT and MRI by 36% and respectively 54% in one study (20).
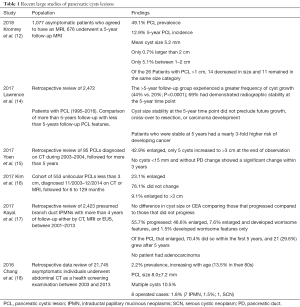
Full table
The description of the small subcentimeter incidental pancreatic cysts is one of the strengths of the Kromrey et al. study. Of the study’s asymptomatic 1,077 subjects who accepted to undergo an MRI, 676 received a 5-year follow-up MRI, with 12.9% newly detected pancreatic cysts and an increase in the number and/or maximum cyst size in 57.1% of the subjects who were found to already have pancreatic cystic lesions on the index MRI. However, these were mostly small cysts, with a mean cyst size 5.2 mm. The almost “1 in 2” 49.1% prevalence is strikingly different from the 2.4% pancreatic cysts prevalence resulted from a prior similar prospective study with only a slightly different study population (21) of 2,803 consecutive asymptomatic patients who underwent an abdominal MRI at a preventive medical examination, where the medial cyst size was 8 mm (range, 2–54 mm) with only 6% of these cysts larger than 2 cm. Another multicenter study (22) that looked at higher risk patients with a strong family history of pancreatic cancer or a predisposing germline mutation that screened 225 asymptomatic adults using computed tomography, magnetic resonance imaging and endoscopic ultrasonography found out that the overall prevalence of a focal pancreatic abnormality in any of the 3 screening tests was 42.6%, with most of the pancreatic mass lesions detected small (mean size 0.55 cm, range, 0.2–3.9 cm), cystic (96%), with patients presenting with multiple lesions (60.7% of those with a cyst). The robust AGA technical review (7) identified 7 studies that reported based on MRI the prevalence of pancreatic cysts to range from 2% to 38%, with an overall prevalence of 15% (95% CI, 7–24%). These are all MRI based studies; the prevalence (23) of pancreatic cysts on autopsies was up to 30% in the 80–89 years old age group and on CT imaging as low as 2.6% (24,25). In the current study, 57% of the patients with pancreatic cysts had an increase in size in 5 years (12) and other studies also suggested that pancreatic cysts still continue to grow 2.2 years later (26) with 11% of them showing delayed growth even after an initial 1-year period of stability. As in other studies, the prevalence of pancreatic cystic lesions was noted to increase with age.
In this observational study by Kromrey et al., important information regarding the cohort is not included in the results that may potentially be important. Of the 1,077 subjects who received MRI/magnetic resonance cholangiopancreatography (MRCP) in this study only 676 received the follow-up in 5 years. It would be of importance to know details of the 401 subjects that did not follow up; and several questions could help clarify the lack of return: (I) how many of these subjects had cysts; (II) what was the size of cyst detected on their baseline scan; and (III) did the subjects follow-up with additional evaluations (i.e., EUS, or imaging earlier than the 5 years) because of either patient concern or high risk features on the initial MRI? Although we are provided with the mean cyst size in Table 1 of this manuscript, a range in size is provided. Standard recommendations and further evaluation are based upon high risk criteria (27) on imaging including cyst size (≥3 cm). We are not provided information on whether those with larger cysts (if there were any) underwent surgery.
The authors expand in their discussion regarding the potential cancer risk in pancreatic cystic lesions and early detection and resection of premalignant lesions is important. However, only three subjects in their 2,333 participants died from pancreatic cancer. Only one of the three subjects with pancreatic cancer had a follow up MRCP; however, we are not told whether any of these three subjects had cysts at baseline. Literature supports that about 85% of those with pancreatic cancer arise from non-cystic lesions called pancreatic intraepithelial neoplasia (PanINs).
Lastly, in is unclear why the 5-year follow-up surveillance time period was chosen. According to the majority of pancreatic cyst guidelines published, most recommend follow-up imaging for cysts without high risk potential within 1 year. Perhaps simple pancreatic cysts in the general population that are incidentally found may be similar to those who have incidental hepatic cysts (8) and renal cysts (28).
The clinical management of pancreatic cystic lesions is currently based on the AGA guideline (29) for incidental asymptomatic cysts and the Sendai guidelines 2006 (30) and their 2012 and 2017 revision—the Fukuoka guidelines (6,27)—for mucinous cystic neoplasms and intraductal papillary mucinous neoplasms, the small percentage of the pancreatic cystic lesions with an established malignant potential. The AGA guidelines recommend MRI surveillance after 1 year and then every 2 years for a total of 5 years for patients with pancreatic cysts without high risk features (size less than 3 cm, without a solid component or a dilated pancreatic duct), with EUS-FNA (endoscopic ultrasound with fine needle aspiration) pursued is one high risk features present and surgery recommended if there are two high risk features. Many studies show that stopping surveillance after 5 years (as recommended by AGA) would miss some cysts that may progress to cancer (14).
The American College of Radiology Incidental Findings in a 2017 expert based white paper (19) expands the follow-up period to 10 years, considering the patient’s age (up to 80 years of age, when surveillance should be stopped if the patient is not a surgical candidate), the pancreatic cyst size and growth and incorporating EUS with FNA as an intervention for higher risk lesions and intervals as short as 6 months for follow-up MRIs. The recommendation to expand the surveillance period to 10 years is based on the finding that even after 5 years of stability pancreatic cysts can still increase in size (14).
The international Fukuoka guideline risk stratify pancreatic cysts based on “high-risk stigmata”(obstructive symptomatology such as jaundice, enhancing mural nodules larger than 5 mm, main pancreatic duct size more than 10 mm) and “worrisome” features (cyst size more than 3 cm, enhancing mural nodule less than 5 mm, thickened enhanced cyst walls, main pancreatic duct size 5–9 mm, abrupt change in the main pancreatic duct diameter with distal pancreatic atrophy, lymphadenopathy, elevated CA19-9 levels, rapid rate of cyst growth more than 5 mm/2 years). Symptomatic cysts (which are not covered by the AGA guideline) or the presence of high risk stigmata should be evaluated clinically for surgical resection, while the presence of worrisome features should be followed for further risk stratification by EUS-FNA, with inconclusive features on EUS-FNA triggering based on cyst size surveillance with EUS alternating with MRI every 3–6 months for cysts larger than 2 cm and the recommendation to consider surgery in young and fit patients instead of prolonged surveillance. In an attempt to increase the yield of EUS exams, there is an evolving role for contrast enhanced harmonic EUS that can demonstrate the presence of blood supply in the mural nodule (31,32), cyst and pancreatic duct lavage (33) and of 19G through the FNA needle cyst wall puncture and micro biopsy (34).
Current research is focusing on risk stratification of the pancreatic cystic lesions (35), molecular and genetic markers of cyst (36) and the role of integrated molecular pathology (37). The AGA recently released a new whitepaper on the role of EUS/FNA for theranostics (38). Others are focusing their attention on developing a “liquid biopsy” (39) using circulating DNA. Circulating miRNAs and metabolites are also promising non-invasive biomarkers that are currently being developed for early detection of pancreatic cancer (40-43).
Despite these shortcomings of this additional study in healthy asymptomatic subjects with incidental pancreatic cysts, the authors conclude that a restrictive follow-up approach can be followed and that screening the general population with imaging for pancreatic cysts to reduce risk of malignancy is not recommended. Based upon the recommendations from the current study and all the prior studies listed (Table 1), researchers can perhaps now stop doing large cohort expensive studies on asymptomatic incidental cysts and turn their attention to factors besides radiographic findings that may identify a cyst-bearing patient at risk for cancer.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Xiaotian Sun (Department of Internal Medicine, Clinic of August First Film Studio, Beijing, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.03.30). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Farrell JJ. Pancreatic Cysts and Guidelines. Dig Dis Sci 2017;62:1827-39. [Crossref] [PubMed]
- Brugge WR. Diagnosis and management of cystic lesions of the pancreas. J Gastrointest Oncol 2015;6:375-88. [PubMed]
- Larson A, Kwon RS. Natural History of Pancreatic Cysts. Dig Dis Sci 2017;62:1770-7. [Crossref] [PubMed]
- Klibansky DA, Reid-Lombardo KM, Gordon SR, et al. The clinical relevance of the increasing incidence of intraductal papillary mucinous neoplasm. Clin Gastroenterol Hepatol 2012;10:555-8. [Crossref] [PubMed]
- Budde C, Beyer G, Kuhn JP, et al. The Clinical and Socio-Economic Relevance of Increased IPMN Detection Rates and Management Choices. Viszeralmedizin 2015;31:47-52. [PubMed]
- Tanaka M, Fernandez-del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology 2012;12:183-97. [Crossref] [PubMed]
- Scheiman JM, Hwang JH, Moayyedi P. American gastroenterological association technical review on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology 2015;148:824-48.e22. [Crossref] [PubMed]
- Marrero JA, Ahn J, Rajender Reddy K, et al. ACG clinical guideline: the diagnosis and management of focal liver lesions. Am J Gastroenterol 2014;109:1328-47; quiz 48. [Crossref] [PubMed]
- Fernandez-Del Castillo C, Tanaka M. Management of pancreatic cysts: the evidence is not here yet. Gastroenterology 2015;148:685-7. [Crossref] [PubMed]
- Singhi AD, Zeh HJ, Brand RE, et al. American Gastroenterological Association guidelines are inaccurate in detecting pancreatic cysts with advanced neoplasia: a clinicopathologic study of 225 patients with supporting molecular data. Gastrointest Endosc 2016;83:1107-17.e2. [Crossref] [PubMed]
- Ma GK, Goldberg DS, Thiruvengadam N, et al. Comparing American Gastroenterological Association Pancreatic Cyst Management Guidelines with Fukuoka Consensus Guidelines as Predictors of Advanced Neoplasia in Patients with Suspected Pancreatic Cystic Neoplasms. J Am Coll Surg 2016;223:729-37.e1. [Crossref] [PubMed]
- Kromrey ML, Bulow R, Hubner J, et al. Prospective study on the incidence, prevalence and 5-year pancreatic-related mortality of pancreatic cysts in a population-based study. Gut 2018;67:138-45. [Crossref] [PubMed]
- Scheiman JM. Pancreatic Cysts - Part 1: Using the American Gastroenterological Association Guidelines for the Management of Pancreatic Cysts-A Practical Approach. Pancreas 2017;46:742-4. [Crossref] [PubMed]
- Lawrence SA, Attiyeh MA, Seier K, et al. Should Patients With Cystic Lesions of the Pancreas Undergo Long-term Radiographic Surveillance?: Results of 3024 Patients Evaluated at a Single Institution. Ann Surg 2017;266:536-44. [Crossref] [PubMed]
- Yoen H, Kim JH, Lee DH, et al. Fate of small pancreatic cysts (<3 cm) after long-term follow-up: analysis of significant radiologic characteristics and proposal of follow-up strategies. Eur Radiol 2017;27:2591-9. [Crossref] [PubMed]
- Kim GE, Shin SS, Kim JW, et al. Incidental, Small (< 3 cm), Unilocular, Pancreatic Cysts: Factors That Predict Lesion Progression during Imaging Surveillance. Korean J Radiol 2017;18:915-25. [Crossref] [PubMed]
- Kayal M, Luk L, Hecht EM, et al. Long-Term Surveillance and Timeline of Progression of Presumed Low-Risk Intraductal Papillary Mucinous Neoplasms. AJR Am J Roentgenol 2017;209:320-6. [Crossref] [PubMed]
- Chang YR, Park JK, Jang JY, et al. Incidental pancreatic cystic neoplasms in an asymptomatic healthy population of 21,745 individuals: Large-scale, single-center cohort study. Medicine (Baltimore) 2016;95:e5535 [Crossref] [PubMed]
- Megibow AJ, Baker ME, Morgan DE, et al. Management of Incidental Pancreatic Cysts: A White Paper of the ACR Incidental Findings Committee. J Am Coll Radiol 2017;14:911-23. [Crossref] [PubMed]
- Khashab MA, Kim K, Lennon AM, et al. Should we do EUS/FNA on patients with pancreatic cysts? The incremental diagnostic yield of EUS over CT/MRI for prediction of cystic neoplasms. Pancreas 2013;42:717-21. [Crossref] [PubMed]
- de Jong K, Nio CY, Hermans JJ, et al. High prevalence of pancreatic cysts detected by screening magnetic resonance imaging examinations. Clin Gastroenterol Hepatol 2010;8:806-11. [Crossref] [PubMed]
- Canto MI, Hruban RH, Fishman EK, et al. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology 2012;142:796-804; quiz e14-5.
- Kimura W, Nagai H, Kuroda A, et al. Analysis of small cystic lesions of the pancreas. Int J Pancreatol 1995;18:197-206. [PubMed]
- Sahani D, Prasad S, Saini S, et al. Cystic pancreatic neoplasms evaluation by CT and magnetic resonance cholangiopancreatography. Gastrointest Endosc Clin N Am 2002;12:657-72. [Crossref] [PubMed]
- Laffan TA, Horton KM, Klein AP, et al. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol 2008;191:802-7. [Crossref] [PubMed]
- Brook OR, Beddy P, Pahade J, et al. Delayed Growth in Incidental Pancreatic Cysts: Are the Current American College of Radiology Recommendations for Follow-up Appropriate? Radiology 2016;278:752-61. [Crossref] [PubMed]
- Tanaka M, Fernandez-Del Castillo C, Kamisawa T, et al. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology 2017;17:738-53. [Crossref] [PubMed]
- Richard PO, Violette PD, Jewett MA, et al. CUA guideline on the management of cystic renal lesions. Can Urol Assoc J 2017;11:E66-73. [Crossref] [PubMed]
- Vege SS, Ziring B, Jain R, et al. American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology 2015;148:819-22; quize12-3.
- Tanaka M, Chari S, Adsay V, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology 2006;6:17-32. [Crossref] [PubMed]
- Ohno E, Hirooka Y, Itoh A, et al. Intraductal papillary mucinous neoplasms of the pancreas: differentiation of malignant and benign tumors by endoscopic ultrasound findings of mural nodules. Ann Surg 2009;249:628-34. [Crossref] [PubMed]
- Harima H, Kaino S, Shinoda S, et al. Differential diagnosis of benign and malignant branch duct intraductal papillary mucinous neoplasm using contrast-enhanced endoscopic ultrasonography. World J Gastroenterol 2015;21:6252-60. [Crossref] [PubMed]
- Sai JK, Nobukawa B, Matsumura Y, et al. Pancreatic duct lavage cytology with the cell block method for discriminating benign and malignant branch-duct type intraductal papillary mucinous neoplasms. Gastrointest Endosc 2013;77:726-35. [Crossref] [PubMed]
- Samarasena JB, Nakai Y, Shinoura S, et al. EUS-guided, through-the-needle forceps biopsy: a novel tissue acquisition technique. Gastrointest Endosc 2015;81:225-6. [Crossref] [PubMed]
- Fonseca AL, Kirkwood K, Kim MP, et al. Intraductal Papillary Mucinous Neoplasms of the Pancreas: Current Understanding and Future Directions for Stratification of Malignancy Risk. Pancreas 2018;47:272-9. [Crossref] [PubMed]
- Deprez PH. Future directions in EUS-guided tissue acquisition. Gastrointest Endosc Clin N Am 2014;24:143-9. [Crossref] [PubMed]
- Al-Haddad MA, Kowalski T, Siddiqui A, et al. Integrated molecular pathology accurately determines the malignant potential of pancreatic cysts. Endoscopy 2015;47:136-42. [PubMed]
- Wani S, Muthusamy VR, McGrath CM, et al. AGA White Paper: Optimizing Endoscopic Ultrasound-Guided Tissue Acquisition and Future Directions. Clin Gastroenterol Hepatol 2018;16:318-27. [Crossref] [PubMed]
- Cohen JD, Javed AA, Thoburn C, et al. Combined circulating tumor DNA and protein biomarker-based liquid biopsy for the earlier detection of pancreatic cancers. Proc Natl Acad Sci U S A 2017;114:10202-7. [Crossref] [PubMed]
- LaConti JJ, Shivapurkar N, Preet A, et al. Tissue and serum microRNAs in the Kras(G12D) transgenic animal model and in patients with pancreatic cancer. PLoS One 2011;6:e20687 [Crossref] [PubMed]
- Alemar B, Gregorio C, Ashton-Prolla P. miRNAs As Diagnostic and Prognostic Biomarkers in Pancreatic Ductal Adenocarcinoma and Its Precursor Lesions: A Review. Biomark Insights 2015;10:113-24. [Crossref] [PubMed]
- Laiakis EC, Mak TD, Anizan S, et al. Development of a metabolomic radiation signature in urine from patients undergoing total body irradiation. Radiat Res 2014;181:350-61. [Crossref] [PubMed]
- Mayerle J, Kalthoff H, Reszka R, et al. Metabolic biomarker signature to differentiate pancreatic ductal adenocarcinoma from chronic pancreatitis. Gut 2018;67:128-37. [Crossref] [PubMed]


