
In vitro and in vivo suppression of hepatocellular carcinoma by Amorphophallus konjac tuber through regulation of survivin and bax
Introduction
Natural compounds from herbs compose a significant part of first-line antitumor drugs, including vincristine, camptothecin and paclitaxel. Since common malignant tumors readily develop chemoresistance, there is high demand for novel antitumor compounds. Natural herbal medicine has become a promising source for novel discoveries, such as curcumin (1), berberine (2), Hedyotis diffusa (3), and decoctions containing undefined bioactive ingredients (4).
There are about 170 species of Amorphophallus worldwide (5), mainly distributed in tropical and subtropical Asia and are used as a food source as well as in traditional Chinese medicine (TCM). The tuber from a major species of Amorphophallus, Amorphophallus konjac [hereafter referred to as Amorphophallus konjac tuber (AKT)], locally produced in Zhejiang, China, has been used in anticancer therapy in TCM for decades (6). Our group has been investigating the efficacy of AKT as well as the underlying mechanism for over 10 years with both clinical practice and laboratory research (7). Recently, an Iranian group has also reported the antitumor effect of AKT (8,9). Thus AKT has become an acknowledged source of natural antitumor compounds. In this article, we show that extracts of AKT could inhibit hepatocellular carcinoma both in vivo and in vitro by induction of cell apoptosis through survivin and bax pathways.
Methods
Herbal medicine and reagents
AKT was purchased from Zhejiang Lin-An Medical Herbs, Co. Ltd. (Batch no. 061208), China. 5-Fluorouracil (5-FU) was from Jinghua Pharmaceutical Group Co. Ltd., China. Other chemical reagents (grade AR) were from Sigma (USA), Amresco (USA) or Aladdin (China).
Preparation of AKT extracts and chemical analysis
Dried AKT was grounded to fine particles, soaked in 8× volume of 95% ethanol overnight, and extracted twice by reverse flow, 2 h each time. The ethanol collected was further extracted by 2× volume of ligarine for twice. The ligarine extract was recovered and concentrated under reduced pressure using a rotary evaporator. The ethanol residuum was extracted by dH2O twice, 10× and 8× volume respectively, the extract was concentrated as well. We did one mega-extraction using 15 kg of AKT and have stored the extracts in 4 °C with desiccants.
Analysis of the composition of the organic extract of AKT
To analyze the rough chemical composition of the AKT extract by ethanol and ligarine, phosphomolybdic acid reaction, acetic anhydride-concentrated sulfuric acid, chloroform-concentrated sulfuric acid, and oil patch reactions were carried out.
Cell culture and cell viability assay
Huh7 and H22 cells were purchased from the Institute of Biochemistry and Cell Biology, Chinese Academy of Science, Shanghai, China. The tumor cell suspension of H22 hepatocellular carcinoma (H22) was induced by C3HA mice, and after subcutaneous transplantation of KM mice, the tumor was transferred to the abdomen. Cells were cultured in DMEM medium (Gibco, USA) containing 10% fetal bovine serum (Gibco, USA) at 37 °C with 5% CO2. The cell viability was measured by a CCK8 kit (Lianke Bio, China) at OD 405 nm. For the viability assays, cells were seeded in 96-well plates, 1×104 cells per well. For short-term treatments, desired drugs, compounds and extracts were added to the wells immediately afterwards and cultured with the cells for 24 h. For long-term treatments, fresh medium with desired reagents were added every 24 h. Then we added 10 µL of CCK-8 solution and NSCLC cells were incubated for an additional 3 h. Cell viability is calculated from the OD values in comparison with the blank. Relative cell viability was calculated as a percentage of untreated controls. The inhibition rate was calculated by the following formula: inhibition rate (%) =100×(ODctrl-ODtreated)/ODctrl; Cell viability (%) = ODtreated/ODctrl ×100. Each treatment condition has been repeated 4 times with at least 3 repeated wells each time.
In vivo H22 tumor model
Use of animals was approved by the institutional ethical committee following the National Research Council Guide the Care and Use of Laboratory Animals. In our experiments, mouse hepatoma H22 cells were intraperitoneally injected in adult male mice (KM strain, 18–20 g, provided by Slac Laboratory Animal, Shanghai, China). The ethical approval number is ZSLL-2009-126. The mice produced tumor ascites after 6–8 days, which is the first generation; then we sacrificed the mice after extraction of ascites, dilution count, intraperitoneal injection of mice again in the abdominal cavity, 6–8 days after the generation of ascites, which is the second generation, at last we repeated the second step to get the third generation of asite cells. The tumor cells were collected by centrifugation and re-suspended in PBS with the concentration of 1×107/mL. Zero point two mL of suspension containing 2×106 cells was seeded to the auxiliary region of each mouse. The mice were randomly divided to 5 groups, 10 in each group. AKT was administered by intragastric gavage at the doses of 100, 200 and 400 mg/kg respectively (0.2 mL/10 g body weight) for 10 days constitutively. 5-FU was used as positive control and 0.9% saline was the negative control. Both the saline and the 5-FU were administered by intragastric gavage with the volume of 0.2 mL/10 g body weight. Weight of the mice were recorded daily prior to drug administration. The mice were sacrificed at the 11th day by cervical luxation.
Tumor tissues were collected from the auxiliary region and weighed. The inhibition rates by different treatments were calculated based on the weight of the tumor masses. Based on following formula: inhibition (%) =100×(Weightctrl-Weighttreated)/Weightctrl.
Flow cytometry analysis
Freshly isolated H22 cells from the tumor mass were collected following tissue sectioning and digestion by trypsin, and were subjected to flow cytometry by apoptosis analysis kit (Beckman Coulter, USA) based on the manufacturer’s instructions. Flow cytometry analysis was performed immediately afterwards (FACSAriaTM, BD Biosciences, USA).
Western blottings
Huh7 cells after desired treatment was scraped, spun down, washed by PBS and resuspended in RIPA buffer with standard protease and phosphatase inhibitors. The lysate was spun at 1,000× g for 10 min and the protein levels from the supernatant were quantified by BCA method (Lianke Bio, China). Proteins from different treatment groups were subjected to SDS-PAGE and transferred to PVDF membranes, which were incubated by primary antibodies (anti-survivin or anti-bax, ZSGB-Bio, Beijing, China; anti-actin, Lianke Bio, China) followed by HRP-conjugated secondary antibodies (Lianke Bio, China). The membranes were developed with an enhanced chemoluminescence kit (Lianke Bio, China). One representative image from three repeated experiments was shown. Quantitative analysis of protein levels determined by band intensities was carried out by ImageJ (v1.48u).
Immunohistochemistry (IHC)
Tumor tissues were fixed in 4% paraformaldehyde, embedded in paraffin and cut into 4 mm sections, then subjected to hematoxylin eosin (HE) staining or IHC. For IHC, tissue sections were incubated with anti-survivin or anti-bax antibodies (both from ZSGB-Bio, Beijing, China). The positive signals were amplified by a biotinylated peroxidase-conjugated streptavidin system (Bio-Genex Laboratories, USA). All specimens were observed and photographed (BX51T-32H01, Olympus, Japan).
Statistical analysis
Each in vitro experimental condition was performed at least 3 times with multiple repeats, and the data were analyzed by SPSS (v13.0). The statistical significance was calculated by one-way ANOVA to compare multiple groups, or by student’s t-test when only two groups were compared. P<0.05 was considered significant, P<0.01 was considered highly significant.
Results
The chemical composition of AKT extracts
After primary separation, we have obtained different of AKT extracts with the following yields calculated from the dry weights: auqa-based extract 223.1% and organic solvent-based extract 5.73%, which constituted a small proportion to the dry weight. By simple chemical reaction analysis, the organic extract was positive in oil patch reaction indicating the presence of volatile oil, and negative for grease, steroids and triterpene.
The in vitro antitumor activity AKT extracts
We have tested the antitumor effect of aqueous and organic extracts of AKT in hepatocellular carcinoma cell lines Huh7 (human) and H22 (mice) (Figure 1A,B). The aqueous extract had very little inhibitory effect, while the organic AKT extract exhibited a strong antitumor effect with IC50 of 35–45 µg/mL based on the inhibition curve (Figure 1A,B). Furthermore, consecutive treatment of 50 µg/mL AKT annihilated Huh7 and H22 within 7 days (Figure 1C,D). In long-term treatments, lower dose of AKT (30 µg/mL) had similar potency as the higher dose (50 µg/mL) to totally inhibit the tumor cell viability within 7 days.
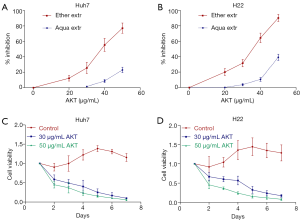
The in vivo antitumor activity of AKT
The organic extract of AKT was used for the in vivo studies. For the in vivo and mechanistic studies, AKT were used considering its relatively high antitumor potency and potentially simpler chemical composition. In Figure 2A,B, after 10 days of continuous AKT treatment by intragastric gavage at 100 mg/kg in KM mice, the hepatocellular carcinoma H22 tumor mass was significantly decreased. HE staining showed that the tumor cells were diffusely distributed with different size, irregular shape, the proportion of nuclear cytoplasm was increased, more common mitosis, interstitial vascular dilatation and congestion, large necrosis in the tumor tissue and lymphocytic infiltration, but there was no significantly difference between control and AKT-treated tissues (Figure 2C). The inhibition rate was 32.4%, comparable with 25 mg/kg 5-FU treatment (35.4%) (Figure 2D). Increase of AKT dose to 200 and 400 mg/kg did not further enhance the inhibition rates. There was no significant difference between the 5-FU group and the AKT groups at different doses.
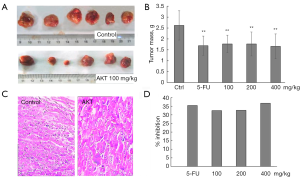
AKT could induce cell apoptosis.
We analyzed the change of apoptotic rate in AKT-treated Huh7 cells. In Figure 3A, flow cytometry was carried out to detect the apoptotic cells induced by AKT after co-staining of annexin V-FITC and PI. After treatment of 24 h, 5-FU induced significant increase of apoptosis; 25 µg/mL AKT did not induce significant increase of apoptotic cells. After cells were treated by 50 and 100 µg/mL AKT, cell apoptosis was significantly increased. Apoptotic rates analyzed by morphological observation and flow cytometry were comparable.
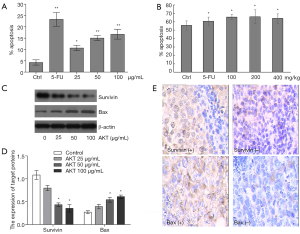
We also analyzed the apoptotic rate in freshly isolated H22 tumor cells grown in vivo by flow cytometry (co-staining of annexin V-FITC and PI). From Figure 3B, the apoptotic rate of isolated H22 tumor cells from the control group was 55.75%, increased to 65.53% after mice were treated by 5-FU (25 mg/kg, intragastric gavage, 10 days), and to 60–66% after treatments of AKT (100, 200 and 400 mg/kg). Increase of AKT from 100 to 400 mg/kg did not further increase the apoptotic rate. Bax and survivin were detected by Western blotting (Figure 3C,D) and IHC (Figure 3E). In Figure 3C, after AKT treatment, levels of survivin had decreased and levels of bax had increased. In Figure 3D, by statistical analysis of repeated Western blottings based on ImageJ, in the AKT 50 and 100 µg/mL groups, levels of survivin was significantly decreased compared with the control group, and levels of bax was significantly increased. In the AKT 25 µg/mL group, there was a trend of decrease of survivin and increase of bax levels, although no statistical significance was revealed. Representative IHC positive and negative images from the AKT treatment group are shown in Figure 3E. The semi-quantitative data is presented in Table 1. In H22 tumors without treatment, 90% samples were survivin positive and 20% samples were bax positive. After treatment by AKT (100, 200 and 400 mg/kg dosage groups combined), the survivin positive rate dropped to 38.5% and the bax positive rate increased to 84.6%. The changes were comparable with the 5-FU group.

Full table
Discussion
In 2014, Ansil et al. used similar extraction method from the tuber of Amorphophallus campanulatus, which this the same species of Amorphophallus konjac, and found antitumor activity of the extract against N-nitrosodiethylamine-induced hepatocellular carcinoma in rats (8) and against human hepatoma cell lines (9). We found the ligarine-based extraction produced potent antitumor extract, while the aquatic extract did not have much effect, suggesting ethanol and ligarine based extraction methods to be more appropriate for the purpose of antitumor treatments of Amorphophallus konjac. From our data, the ethanol-ligarine extract of AKT contained various organic compounds including nitrogen and sulfur-containing compounds. Further separation and identification of these components are to be carried out in future studies by nuclear magnetic resonance (NMR) etc. The bioactive chemical is to be identified. However, according to the holistic theory of TCM, it is also likely that the efficacy is due to the combinational use of multiple ingredients.
A TCM decoction contains hundreds of different chemical components and modulate many molecular targets simultaneously to result in complicated cascades of cellular events. Qing-Yi-Hua-Ji formula, a decoction with AKT as one of the major ingredients, could increase the survival of late stage pancreatic cancer patients (10), and has been reported to affect multiple downstream targets including Ski (11), Notch-4 and Jagged-1 (12). We found AKT could induce apoptosis both in vitro and in vivo and regulated the levels of survivin and bax both in vitro and in vivo. Recent studies have been reported that Amorphophallus tuber extract in inhibits the activity of cytotoxicity and promoted apoptosis in human colon carcinoma cells and hepatoma cells (9,13). Survivin belongs to the family of inhibitors of apoptosis proteins (IAP) that regulate cell death by inhibition of caspase activation. It is expressed only in cells in the G2/M phase, but absent in differentiated cells (14,15). Survivin is highly expressed in most human tumors and fetal tissues, and has thus become a biomarker and a therapeutic target in cancer (16). From our data, AKT could decrease the levels of the anti-apoptotic proteins survivin and simultaneously increase the levels of the pro-apoptotic proteins bax. AKT could regulate apoptosis through the IAP family of proteins, although was not necessarily dependent on them.
Amorphophallus konjac is well tolerated as a food source in some countries. Among the 94 organic compounds identified from konjac powder, none was toxic by the standards of USFDA (17). It contains glucomannans, which are potentially applicable in clinical use against obesity and diabetes. They can decrease body weight through regulating food absorption in the gut, and have been registered in clinical trials in adults (18) and in children (18,19). The tuber part of Amorphophallus konjac of has been used in cancer treatment in TCM for decades. Glucomannans, the most studied bioactive component from konjac powder, are typically extracted by water followed by coagulation by ethanol, which produces much higher yields compared with the organic solvents-based isolation methods (20). Our data and previous report indicate that the potent fractions with antitumor activity from AKT are contained in the ethanol-ligarine extract. Although the inhibition was dose-dependents in the 0–50 µg/mL range at the 24 h time-point, the inhibition reached saturation at low doses when using in long-term treatment. From the in vivo experiments, increase of AKT from 100 to 400 mg/kg also resulted in similar inhibition rates. Although safe to consume, our data suggest that AKT-based medication in relatively smaller doses would be efficient to achieve desired effects.
Overall, our findings strongly support the feasibility to isolate novel antitumor compounds from the Amorphophallus konjac, which could be used as an alternative or in combinational regimen in the clinic against hepatocellular carcinoma.
Acknowledgments
We thank Dr. Xi Chen from Zhejiang University, Children’s Hospital for her useful criticism during preparation of the manuscript.
Funding: This research is funded by the Foundation from Zhejiang Provincial Natural Science Foundation of China (grant no. LY16H270006) and Research Foundation of Zhejiang Chinese Medical University (grant no. 2015ZG08).
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.01.26). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Use of animals was approved by the institutional ethical committee following the National Research Council Guide the Care and Use of Laboratory Animals. The ethical approval number is ZSLL-2009-126.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kumar G, Mittal S, Sak K, et al. Molecular mechanisms underlying chemopreventive potential of curcumin: Current challenges and future perspectives. Life Sci 2016;148:313-28. [Crossref] [PubMed]
- Jin P, Zhang C, Li N. Berberine exhibits antitumor effects in human ovarian cancer cells. Anticancer Agents Med Chem 2015;15:511-6. [Crossref] [PubMed]
- Dong Q, Ling B, Gao B, et al. Hedyotis diffusa water extract diminished the cytotoxic effects of chemotherapy drugs against human breast cancer MCF7 cells. Nat Prod Commun 2014;9:699-700. [PubMed]
- Fang LH, Wang RP, Hu SY, et al. The effect of tou nong san on transplanted tumor growth in nude mice. Evid Based Complement Alternat Med 2015;2015:518454 [PubMed]
- Chua M, Baldwin TC, Hocking TJ, et al. Traditional uses and potential health benefits of Amorphophallus konjac K. Koch ex N.E. Br. J Ethnopharmacol 2010;128:268-78. [Crossref] [PubMed]
- Committee CP. Pharmacopoeia of the People's Republic of China. Beijing: China Chemical Industry Press, 2010.
- Chen PF, Liu LM. Observation on the Anti-tumor effects and Induction carcinoma cell’s Apoptosis of She Liu Gu. Chines J Bas Med Tcm 2000;6:30-3.
- Ansil PN, Nitha A, Prabha SP, et al. Curative effect of Amorphophallus campanulatus (Roxb.) Blume. tuber on N-nitrosodiethylamine- induced hepatocellular carcinoma in rats. J Environ Pathol Toxicol Oncol 2014;33:205-18. [Crossref] [PubMed]
- Ansil PN, Wills PJ, Varun R, et al. Cytotoxic and apoptotic activities of Amorphophallus campanulatus tuber extracts against human hepatoma cell line. Res Pharm Sci 2014;9:269-77. [PubMed]
- Liu L, Wu L, Lin S. Therapeutic evaluation on advanced pancreatic cancer treated by integrative Chinese and western medicine: clinical analysis of 56 cases. Chin J Integr Med 2003;9:39-43. [Crossref]
- Wang P, Chen Z, Meng ZQ, et al. Ski acts as therapeutic target of qingyihuaji formula in the treatment of SW1990 pancreatic cancer. Integr Cancer Ther 2010;9:50-8. [Crossref] [PubMed]
- Xu Y, Zhu F, Xu S, et al. Anti-tumor effect of the extract from qingyihuaji formula on pancreatic cancer by down-regulating Notch-4 and Jagged-1. J Tradit Chin Med 2015;35:77-83. [Crossref] [PubMed]
- Ansil PN, Wills PJ, Varun R, et al. Cytotoxic and apoptotic activities of Amorphophallus campanulatus (Roxb.) Bl. tuber extracts against human colon carcinoma cell line HCT-15. Saudi J Biol Sci 2014;21:524-31. [Crossref] [PubMed]
- Altieri DC. Validating survivin as a cancer therapeutic target. Nat Rev Cancer 2003;3:46-54. [Crossref] [PubMed]
- Sah NK, Khan Z, Khan GJ, et al. Structural, functional and therapeutic biology of survivin. Cancer Lett 2006;244:164-71. [Crossref] [PubMed]
- Altieri DC. Survivin, cancer networks and pathway-directed drug discovery. Nat Rev Cancer 2008;8:61-70. [Crossref] [PubMed]
- Chen X, Yuan LQ, Li LJ, et al. Suppression of gastric cancer by extract from the tuber of amorphophallus konjac via induction of apoptosis and autophagy. Oncol Rep 2017;38:1051-8. [Crossref] [PubMed]
- Zalewski BM, Chmielewska A, Szajewska H. The effect of glucomannan on body weight in overweight or obese children and adults: a systematic review of randomized controlled trials. Nutrition 2015;31:437-42. [Crossref] [PubMed]
- Zalewski BM, Szajewska H. Effect of glucomannan supplementation on body weight in overweight and obese children: protocol of a randomised controlled trial. Bmj Open 2015;5:e007244 [Crossref] [PubMed]
- Harmayani E, Aprilia V, Marsono Y. Characterization of glucomannan from Amorphophallus oncophyllus and its prebiotic activity in vivo. Carbohydr Polym 2014;112:475-9. [Crossref] [PubMed]


