
Primary hepatic neuroendocrine tumors: retrospective analysis of seven cases and literature review
Introduction
Neuroendocrine tumors (NETs) are a group of highly heterogeneous diseases derived from the diffuse neuroendocrine system which are thought to be very rare (1). However, with further understanding of these diseases and improvement in the technologies available for their clinical diagnosis, larger numbers of NETs are being identified in developed countries. According to the American National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) database, the incidence of gastroenteropancreatic NETs has increased in developed countries over the last 30 years (2,3). Brandolini et al. have shown that number of NETs continued to increase (4).
NETs mostly arise not only in the gastrointestinal tract and pancreas (66%) but also in the bronchopulmonary system (31%) (5). Primary hepatic neuroendocrine tumors (PHNETs), by comparison, are extremely rare with only about 150 cases having been reported in the English-language literature (6). There is still no specific diagnostic criteria on PHNETs. Some reports (6,7) suggested that the diagnosis of PHNETs relies on pathological confirmation and imaging methods. Immunohistochemical examination has shown that NETs are characterized by specific markers, including chromogranin A (CgA) and synaptophysin (Syn) (8,9). In addition, imaging methods, including computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography-CT (PET-CT), are useful to exclude extrahepatic NETs (6,7). Surgical resection is the only treatment that may be capable of achieving a cure (10-12). There are various other treatment measures available such as radiofrequency ablation (RFA), transcatheter arterial chemoembolization (TACE), somatostatin analogs and chemotherapy. However, there is still no consensus on treatment of PHNETs.
Due to the rarity of PHNETs, their clinical features, diagnosis and effective treatment strategies are not well understood. In this study, we combined the clinicopathological data retrospectively analyzed from seven PHNET patients with literature review to explore them.
Methods
Patients
The clinical data from seven PHNETs cases that were admitted to the Cancer Institute & Hospital, Chinese Academy of Medical Sciences, during the period April 2009 to November 2017, were collected by a medical records management system. The diagnosis of PHNETs was confirmed by postoperative pathology identified NETs and preoperative and postoperative imaging examination to exclude primary extrahepatic NETs. In order to improve accuracy of diagnosis, all patients were requested to receive regular imaging examination including CT or MRI after operation. At the same time, follow-up examinations of all patients were conducted. The patients provided informed consent for data analysis. The study protocol was approved by the Institutional Review Board of the Cancer Hospital of Chinese Academy of Medical Sciences.
Imaging examination
Initially, patients underwent different imaging examinations including ultrasound (US), CT, MRI, 18F-fluorodeoxyglucose (18F-FDG) PET-CT to evaluate the state of disease, lesion size, number, shape, location and metastasis, and tumor characteristics such as necrosis and hemorrhage. After treatment, patients underwent imaging examination to evaluate treatment response and recurrence. At the same time, preoperative and postoperative regular imaging examination is requested to exclude primary extrahepatic NETs. Two experienced digestive system imaging specialists reviewed the images and reached a consensus.
Treatment
A multi-disciplinary team of surgeons, medical oncologists, radiation oncologists, radiologists, and pathologists formulated comprehensive individualized treatment strategies for all the patients. The means of treatment include surgical resection, RFA, TACE and systemic chemotherapy.
Pathological analysis
Tissue slices stained with hematoxylin-eosin (H&E) and immunohistochemical staining agents including CgA, Syn, CD56, hepatocyte, carcinoembryonic antigen (CEA) and alpha-fetoprotein (AFP). In this study, Syn and CgA were examined to confirm neurosecretory character and hepatocyte was used to exclude hepatocellular origin. Pathological staging of PHNET patients was conducted using the 2010 World Health Organization (WHO) Classification of Tumors of the Digestive System and the 2015 National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology. Three pathologists who were familiar with NETs independently and retrospectively reviewed the pathological diagnosis.
Results
Clinical features
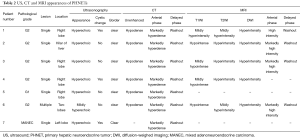
After excluding 12 metastatic hepatic NETs by imaging examination, seven patients were diagnosed with PHNETs. During the period of the study, 6,624 primary hepatic tumors and 905 NETs were diagnosed. PHNETs accounted for 0.11% and 0.77% of these, respectively. None of the patients had a medical history of hepatitis or liver cirrhosis. The PHNET patients consisted of two men and five women with an average age of 52.3 years (range, 41−62 years). Four of the patients were asymptomatic and three were presented with abdominal pain. Physical examination did not detect any obvious abnormality. The serum AFP level and CEA level were elevated in five patients. Biochemical assay of tumor markers showed that five cases (5/5) were AFP negative and five cases (5/5) were CEA negative. Liver function tests demonstrated normal serum levels of alanine aminotransferase, aspartate aminotransferase, and total bilirubin in all the patients (Table 1).
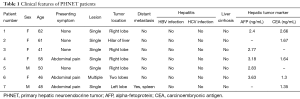
Full table
Appearance on imaging
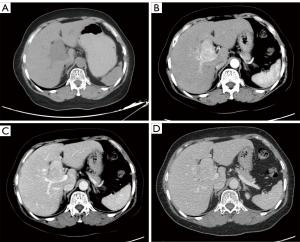
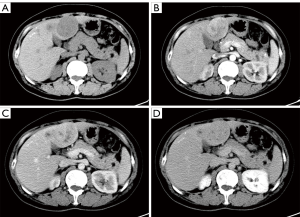
Seven patients underwent pre-treatment CT examination. Single solid intrahepatic lesions with clear boundaries ± cystic areas were detected in five of them. Multiple intrahepatic masses located in two liver lobes with unclear boundaries were detected in one patient, and a huge splenic mass (~15 cm × 11 cm) that was closely related to the left liver lobe was found in another patient. Postoperative pathology confirmed mixed adenoneuroendocrine carcinoma (MANEC) of the left liver lobe with splenic metastasis in this patient. While the standard CT scan showed single or multiple intrahepatic hypoechoic masses or nodules, contrast-enhanced CT showed markedly heterogeneous enhancement of the masses or nodules in the arterial phase, with washout in the portal venous and delayed phases (Figures 1,2).
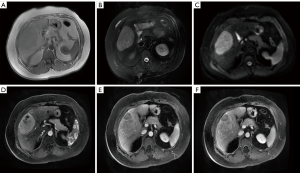
Five patients underwent pre-treatment MRI examination. Among them, four single intrahepatic lesions were detected, while multiple intrahepatic lesions were detected in one patient. The scans showed heterogeneous and hypointense masses with a lower cystic signal and a higher hemorrhagic signal area on T1-weighted MRI, a high signal intensity in T2-weighted MRI, and restricted diffusion on diffusion-weighted (DW)-MRI. On Dynamic MR imaging, the masses showed markedly annular or heterogeneous enhancement in the arterial phase with washout in the portal venous and delayed phases (Figure 3).
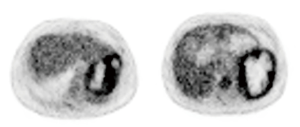
After one cycle of cisplatin and etoposide treatment, one patient underwent preoperative 18F-FDG PET-CT, 18F-FDG PET-CT showed there were two low density masses (12.6 cm × 9.1 cm and 5 cm × 4 cm) with low or high radiative defects representing necrosis in their centers, marginally greater uptake by the spleen, and a low-density mass with normal uptake in the right liver lobe (Table 2 and Figure 4).
Full table
All patients underwent regular CT examination or MRI examination after the operation, which identified no extrahepatic tumors at the end of the follow-up period ranging from 10 to 87 months.
Treatment
Surgical resection was performed in all the patients in this group. The surgical approaches were as follows: two cases underwent liver sectoriectomy, one underwent combined liver segmentectomy, one underwent limited liver resection, one underwent liver limited resection, splenectomy, and simultaneous partial diaphragmatic resection, one underwent right hemihepatic resection, and the last underwent multiple hepatic tumor resection and intraoperative RFA. Zero resection was achieved in all patients. Of the seven cases, two had received preoperative systemic chemotherapy, one had undergone preoperative TACE and RFA, one had undergone TACE alone, and one underwent RFA intraoperatively (Table 3).
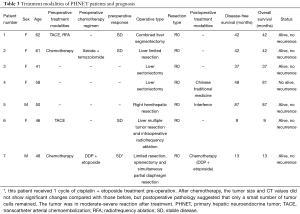
Full table
Pathological features
The seven patients were all confirmed to have NETs by post-surgical pathology. According to the 2010 WHO Classification of Tumors of the Digestive System and the 2015 NCCN Clinical Practice Guidelines in Oncology, there was one case of low-grade NET (G1), five cases of intermediate grade NET (G2), and one case of primary hepatic MANEC. Six patients had a single intrahepatic lesion and one had multiple intrahepatic lesions. Six patients did not show distant metastasis, but one did have extrahepatic metastasis (splenic metastases). The size of the tumors ranged from 0.2 to 14 cm. On immunohistochemistry all of the tumors were CgA positive, 6 (85.7%) were Syn positive, 5 (71.4%) were CD56 positive, 6 (85.7%) were hepatocyte negative, 1 (14.3%) was hepatocyte positive (hepatic MANEC), all were CEA negative, and 6 (85.7%) were AFP negative (Table 4 and Figure 5).
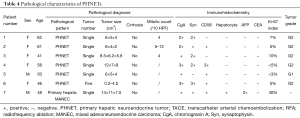
Full table
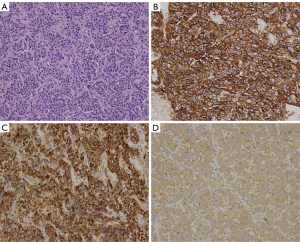
Prognosis
At the end of the follow-up period ranging from 9 to 87 months, six patients survived and one died because of tumor recurrence. One patient with postoperative recurrence did not receive any standard treatment and still survived for 33 months after recurrence. One patient with multiple PHNETs received RFA intraoperatively and there had been no recurrence at the ablation site for 9 months afterwards. The longest postoperative survival time and the longest disease-free survival (DFS) time were all 87 months (Table 3).
Discussion
NETs are highly heterogeneous tumors derived from the diffuse neuroendocrine system (1) that mostly arise in the gastrointestinal tract, pancreas (66%) and bronchopulmonary system (31%) (5). By comparison, PHNETs are extremely rare (13). PHNETs were first described by Edmondson in 1958 (14) and by 2012 around 150 cases had been reported in the English-speaking literature (6). During our study period, 6,624 primary hepatic tumors and 905 NETs were identified. PHNETs accounted for 0.11% and 0.77% of these, respectively. The clinical features, diagnosis and treatment strategies of PHNETs are not well understood. Therefore, we combined our data with literature information (Table 5) to summarize characteristics of PHNETs as follows to make clinicians pay more attention to this disease, and get further understanding in this field.
Full table
The origin of PHNETs is not clear, but there are three hypotheses. First, tumor cells arise from the epithelium of the intrahepatic biliary tract; second, ectopic pancreatic or adrenal tissues located in the liver give rise to PHNETs; and third, PHNETs originate from malignant hepatic stem cell precursors through neuroendocrine differentiation (20).
PHNETs mainly arise between 40 and 60 years of age and no sex bias has been identified (6,7,10,12,15-19) (Table 5). The PHNET patients usually have no specific symptoms and only 6.8% of cases present a typical carcinoid syndrome, characterized by skin flushing and diarrhea (21). Therefore, early diagnosis of PHNETs relying on symptoms is difficult. Furthermore, in contrast to hepatocellular carcinomas (HCCs), PHNETs are not associated with cirrhosis or hepatitis, and hepatic tumor markers including AFP and CEA are within the normal range in the presence of PHNETs (6,7,10,12,15-19) (Table 5). Our results in this study are consistent to these conclusions.
On imaging, it is difficult to differentiate PHNETs with a rich blood supply from the hepatic artery from other primary or secondary hepatic tumors with a rich blood supply from the hepatic artery, such as HCC, hepatic adenoma and focal nodular hyperplasia (FNH) (22). However, one report (7) suggested dynamic CT or MR imaging of PHNETs show marked enhancement in the arterial phase with washout in the portal venous and delayed phase, but enhancement in PHNETs is still higher than that of the surrounding liver tissue. Some studies (7,22) demonstrated CT and MRI can reflect tumor grade and pathological features of PHNETs. Our study suggested MRI show heterogeneous and hypointense masses with lower cystic and hemorrhagic signal areas on T1-weighted MRI, high signal intensity on T2-weighted MRI, and restricted diffusion on DW-MRI, and dynamic CT or MR imaging is consistent to the report (7). Therefore, PHNETs have some characteristics on imaging appearance.
Approximately 90% of NETs express somatostatin receptors (SSTRs), which permit functional imaging examination (23). SSTR scintigraphy (SRS) has been widely used in the diagnosis of NETs in recent years. 68Ga-Dota-DPhe1 and Tyr3-octreotide (DOTATATE) PET-CT had a rate of 95.2% in the detection of primary and metastatic gastro-entero-pancreatic NETs (24). In addition, 18F-FDG PET-CT was positive in 57% of patients with NET grade 1 and 66% of patients with NET grade 2 (25). In diagnosis of PHNETs, SSTR PET-CT and 18F-FDG PET-CT are useful to find PHNETs and exclude extrahepatic NETs. In our study, one patient with pathologically diagnosed MANEC underwent 18F-FDG PET-CT (Figure 4). To the best of our knowledge, no previous cases of primary hepatic MANEC with splenic metastasis detected using 18F-FDG PET-CT imaging have been reported. However, the cost of SSTR PET-CT and 18F-FDG PET-CT is very high, which is about ten times of that of CT, and they cannot even be accessible in most hospitals. It is very difficult to request every suspected PHNET patient to undergo SSTR PET-CT and 18F-FDG PET-CT. On the other hand, due to the high heterogeneity and complexity of PHNETs, the hepatic tumors of most patients were initially considered HCCs or cholangiocellular carcinomas, not NETs, many patients will miss the opportunity to check. According to our statistics (Table 5), only 16% PHNET patients received PET-CT.
There is still no specific diagnostic criteria on PHNETs. Some reports (6,7) suggested that the diagnosis of PHNETs relies on pathological confirmation and imaging methods. Pathological and immunohistochemical evaluation is required for the diagnosis of PHNETs. Histopathological diagnosis of PHNETs should include routine H&E staining and the detection of immunohistochemical markers. Routine H&E staining is not specific for NETs, but it is helpful in classifying tumor grade according to the irregularity and hyperchromicity of the nuclei of tumor cells. It is generally accepted that expression of CgA, Syn and neuron-specific enolase represents definitive evidence for the diagnosis (8,9). In our study, all cases were CgA positive and 6 cases (85.7%) were Syn positive, which is consistent with the previous reports. However, it is difficult to distinguish PHNETs from extrahepatic NETs based on pathological evidence alone. Thorough imaging examination prior to surgery, regular imaging examination during follow-ups after surgery are important in detecting extrahepatic neuroendocrine tumors. Functional tests such as SSTR PET-CT and 18F-FDG PET-CT are helpful for diagnosis, but the high cost and poor accessibility limit its utility. Patients in our study underwent regular CT examination or MRI examination after the operation, which identified no extrahepatic tumors at the end of the follow-up period ranging from 9 to 87 months.
There is still no consensus on treatment of PHNETs. However, some reports suggested surgical resection is the only treatment that may be capable of achieving a cure (10-12). Massive researches reported that the resectability rate and OS are satisfactory in spite of recurrence (6,7,10,12,15-19) (Table 5). Nowadays, for surgical resection of PHNETs, the longest postoperative survival time was 107 months and the longest DFS time was 98 months (6,7,10,12,15-19) (Table 5). In our study, all the patients underwent surgical resection and six remained alive without recurrence, one patient with postoperative recurrence did not receive any standard treatment and still survived for 33 months after recurrence. The longest postoperative survival time and the longest DFS time were all 87 months. These results suggest surgical resection may lead to a good prognosis for PHNET patients. There are various other treatment measures available such as RFA, TACE and chemotherapy. RFA is a local treatment method for PHNETs, which has been implemented when three or fewer tumors are present with a diameter of ≤5 cm (26) or when small tumors with diameters ≤3 cm are present (17). In our study, one patient with multiple PHNETs received RFA intraoperatively and there had been no recurrence at the ablation site for 9 months. TACE can be used for the cytoreduction of NETs because NETs are hypervascular and sensitive to ischemia (27,28). For PHNETs, TACE is a preferred non-operative treatment that results in a good prognosis (29). For PHNET patients with multiple masses or metastases are ineligible for surgical resection. Chemotherapy is available for PHNETs, but its effect has not been well characterized. Cytotoxic drugs are a good choice for tumors with a high proliferation index, but the most highly recommended protocol involves a combination of chemotherapy agents, such as 5-fluorouracil (5-FU), etoposide, and cisplatin (30). For example, the PHNET patients who received chemotherapy with 5-FU plus etoposide plus cisplatin showed partial responses (6). In this study, one patient with primary hepatic MANEC received one cycles of cisplatin and etoposide treatment before surgery. After chemotherapy, postoperative pathology suggested that only a small number of tumor cells remained. The tumor showed a moderate to severe reaction after treatment.
In summary, PHNETs are extremely rare. PHNETs mainly arise between 40 and 60 years of age with no sex bias. Patients usually have no specific symptoms. On imaging, it is difficult to differentiate them from other tumors with a rich blood supply, such as HCC, hepatic adenoma and FNH. However, when patients are with no medical history of hepatitis or liver cirrhosis and with normal serum AFP levels, but with a large dominant hypervascular hepatic mass accompanied by a lower cystic signal and a higher hemorrhagic signal area on T1-weighted MRI, with marked enhancement on arterial phase, rapid washout on portal venous phase and capsular enhancement on dynamic MR imaging or dynamic CT imaging and restricted diffusion on DW-MRI, PHNETs should be suspected. Functional tests such as SRS, 68Ga-DOTATATE, and 18F-FDG PET-CT are recommended for diagnosis, but the high cost and poor accessibility limit its utility. The diagnosis of PHNETs relies on pathological confirmation and imaging examination to exclude extrahepatic neuroendocrine tumors. For patients confirmed to have PHNETs, surgery-centered comprehensive individualized treatment strategies should be formulated. For patients with locally resectable lesions, surgical resection is the first choice, which not only leads to a good prognosis but also provides a pathological diagnosis. However, these conclusions need more evidence to confirm.
Acknowledgments
We would like to thank all the volunteers who took part in this study.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.04.07). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the Institutional Review Board of the Cancer Hospital of Chinese Academy of Medical Sciences (No. 17-194/1450). The clinical samples were obtained with informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hu W, Feng Z, Modica I, et al. Gene Amplifications in Well-Differentiated Pancreatic Neuroendocrine Tumors Inactivate the p53 Pathway. Genes Cancer 2010;1:360-8. [Crossref] [PubMed]
- Lawrence B, Gustafsson BI, Chan A, et al. The Epidemiology of Gastroenteropancreatic Neuroendocrine Tumors. Endocrinol Metab Clin North Am 2011;40:1-18. vii. [Crossref] [PubMed]
- Yao JC, Hassan M, Phan A, et al. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26:3063-72. [Crossref] [PubMed]
- Brandolini J, Bertolaccini L, Pardolesi A, et al. Surgical treatment of synchronous multiple neuroendocrine lung tumours (case series): is more always better? Ann Transl Med 2017;5:423. [Crossref] [PubMed]
- Wang HB, Yang Y, Fan XW, et al. Thymic neuroendocrine tumors: an analysis of 18 cases and a literature review. Transl Cancer Res 2016;5:789-96. [Crossref]
- Park CH, Chung JW, Jang SJ, et al. Clinical features and outcomes of primary hepatic neuroendocrine carcinomas. J Gastroenterol Hepatol 2012;27:1306-11. [Crossref] [PubMed]
- Chen Z, Xiao H, Ramchandra P, et al. Imaging and pathological features of primary hepatic neuroendocrine carcinoma: An analysis of nine cases and review of the literature. Oncol Lett 2014;7:956-62. [Crossref] [PubMed]
- Campana D, Nori F, Piscitelli L, et al. Chromogranin A: is it a useful marker of neuroendocrine tumors? J Clin Oncol 2007;25:1967. [Crossref] [PubMed]
- Pelosi G, Sonzogni A, Harari S, et al. Classification of pulmonary neuroendocrine tumors: new insights. Transl Lung Cancer Res 2017;6:513-29. [Crossref] [PubMed]
- Hwang S, Lee YJ, Lee SG, et al. Surgical treatment of primary neuroendocrine tumors of the liver. J Gastrointest Surg 2008;12:725-30. [Crossref] [PubMed]
- Sotiropoulos GC, Charalampoudis P, Delladetsima I, et al. Surgery for giant primary neuroendocrine carcinoma of the liver. J Gastrointest Surg 2014;18:839-41. [Crossref] [PubMed]
- Yalav O, Ülkü A, Akçam TA, et al. Primary hepatic neuroendocrine tumor: Five cases with different preoperative diagnoses. Turk J Gastroenterol 2012;23:272-8. [Crossref] [PubMed]
- Fenoglio LM, Severini S, Ferrigno D, et al. Primary hepatic carcinoid: a case report and literature review. World J Gastroenterol 2009;15:2418-22. [Crossref] [PubMed]
- Edmondson HA. Tumors of the liver and intrahepatic bile ducts. In: Atlas of tumor pathology. Washington, DC: Armed Forces Institute of Pathology, 1958:18.
- Pilichowska M, Kimura N, Ouchi A, et al. Primary hepatic carcinoid and neuroendocrine carcinoma: clinicopathological and immunohistochemical study of five cases. Pathol Int 1999;49:318-24. [Crossref] [PubMed]
- Donadon M, Torzilli G, Palmisano A, et al. Liver resection for primary hepatic neuroendocrine tumours: report of three cases and review of the literature. Eur J Surg Oncol 2006;32:325-8. [Crossref] [PubMed]
- Huang YQ, Xu F, Yang JM, et al. Primary hepatic neuroendocrine carcinoma: clinical analysis of 11 cases. Hepatobiliary Pancreat Dis Int 2010;9:44-8. [PubMed]
- Li RK, Zhao J, Rao SX, et al. Primary hepatic neuroendocrine carcinoma: MR imaging findings including preliminary observation on diffusion-weighted imaging. Abdom Imaging 2013;38:1269-76. [Crossref] [PubMed]
- Wang LM, An SL, Wu JX. Diagnosis and therapy of primary hepatic neuroendocrine carcinoma: clinical analysis of 10 cases. Asian Pac J Cancer Prev 2014;15:2541-6. [Crossref] [PubMed]
- Gravante G, De Liguori Carino N, Overton J, et al. Primary carcinoids of the liver: a review of symptoms, diagnosis and treatments. Dig Surg 2008;25:364-8. [Crossref] [PubMed]
- Quartey B. Primary Hepatic Neuroendocrine Tumor: What Do We Know Now? World J Oncol 2011;2:209-16. [PubMed]
- Wang LX, Liu K, Lin GW, et al. Primary hepatic neuroendocrine tumors: comparing CT and MRI features with pathology. Cancer Imaging 2015;15:13. [Crossref] [PubMed]
- Hasegawa S, Kobayashi N, Tokuhisa M, et al. Clinical Usefulness of Somatostatin Receptor Scintigraphy in Japanese Patients with Gastroenteropancreatic Neuroendocrine Tumors. Digestion 2017;96:13. [Crossref] [PubMed]
- Sadowski SM, Neychev V, Millo C, et al. Prospective Study of 68Ga-DOTATATE Positron Emission Tomography/Computed Tomography for Detecting Gastro-Entero-Pancreatic Neuroendocrine Tumors and Unknown Primary Sites. J Clin Oncol 2016;34:588. [Crossref] [PubMed]
- Severi S, Nanni O, Bodei L, et al. Role of 18FDG PET/CT in patients treated with 177Lu-DOTATATE for advanced differentiated neuroendocrine tumours. Eur J Nucl Med Mol Imaging 2013;40:881-8. [Crossref] [PubMed]
- Boonsirikamchai P, Loyer EM, Choi H, et al. Planning and follow-up after ablation of hepatic tumors: imaging evaluation. Surg Oncol Clin N Am 2011;20:301-15. [Crossref] [PubMed]
- Kress O, Wagner HJ, Wied M, et al. Transarterial Chemoembolization of Advanced Liver Metastases of Neuroendocrine Tumors – A Retrospective Single-Center Analysis. Digestion 2003;68:94-101. [Crossref] [PubMed]
- Varker KA, Martin EW, Klemanski D, et al. Repeat transarterial chemoembolization (TACE) for progressive hepatic carcinoid metastases provides results similar to first TACE. J Gastrointest Surg 2007;11:1680-5. [Crossref] [PubMed]
- Vijgen S, Terris B, Rubbia-Brandt L. Pathology of intrahepatic cholangiocarcinoma. Hepatobiliary Surg Nutr 2017;6:22-34. [Crossref] [PubMed]
- Maroun J, Kocha W, Kvols L, et al. Guidelines for the diagnosis and management of carcinoid tumours. Part 1: The gastrointestinal tract. A statement from a Canadian National Carcinoid Expert Group. Curr Oncol 2006;13:67-76. [PubMed]


