
Upregulated GP73 expression and downregulated NLRP3 expression in liver cancer tissues correlate with patient’s survival
IntroductionOther Section
Liver cancer is the second leading cause of tumor-related deaths worldwide and has an incidence of around 850,000 new cases per year about half of them in China (1,2). Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer. HCC occurs usually in people with cirrhosis caused by hepatitis B or hepatitis C infection, alcohol intake and ingestion of the fungal metabolite aflatoxin B1. The normal outcome is poor, because only 10–20% of HCC can be resected completely using surgery (3). If the tumor cannot be completely resected, the patients are usually died within 3 to 6 months (4). However, the poor prognosis could be improved due to the detection of early-stage tumors (5). Hepatectomy, transplantation or local ablation are effective therapies at early stage but only chemoembolization and sorafenib have shown survival benefits at developed stages (6). Therefore, it will be important to develop new techniques for diagnosis and prognostic prediction in order to get more curative treatment for patients with HCC.
Golgi protein 73 (GP73) is a 73-kD transmembrane glycoprotein that located in the cis-Golgi complex. Recent years, GP73 was used in clinical diagnosis as a serum biomarker for HCC (7). The largest cohort research of GP73, our former study revealed that GP73 had a sensitivity of 74.6% and a specificity of 97.4% for HCC diagnosis. Further studies had shown that the serum GP73 had no relationship with clinicopathologic features but the GP73 expressions in HCC tissues had correlation with tumor size, differentiation and prognosis (8). We also found that GP73 could increase the proliferation and mobility of HCC cell lines and cancer progress in mice (9). This effect may be associated with inflammatory response (10).
Nucleotide-binding oligomerization domain-containing protein (NOD)-like receptor family pyrin domain containing 3 (NLRP3) is one of the recent research focus for several diseases (11), and it has been revealed to regulate various inborn and adaptive immune responses (12). NLRP3 protein expression also involve in many kind of tumor progression (13). In this study, we tried to investigate the relationship between NLRP3 expressions in HCC tissues and clinical features of HCC patients, and to evaluate GP73 and NLRP3 expressions in HCC tissues whether be useful for predicting the prognosis as an auxiliary marker.
MethodsOther Section
Patients and samples
In this study, 65 subjects who received liver resection in the Department of Liver Surgery of Peking Union Medical College Hospital (PUMCH) were enrolled from April 2010 to March 2014. Fifty-four HCC tissues and matched paracarcinomatous liver (PCL) tissues from patients with HCC and 11 healthy liver (HL) tissues from patients with hepatic hemangioma were acquired in the operation theater. PCL tissue was collected 2–5 cm away from the tumor border. These specimens were frozen in liquid nitrogen immediately and then stored in −80 °C until the further testing.
The diagnosis of HCC based on histopathology. The clinicopathologic parameters, including age, gender, etiological factor, AFP level, multiplicity, tumor size, tumor differentiation, vein invasion, recurrence, and survival time, were recorded. The judgment of vein invasion and recurrence were according to the previously study (8,14).
The study protocol was approved by the institutional ethical committee of PUMCH with No. PUMCH-2009-079, and written informed consent was obtained from each patient before enrolling.
GP73 and NLRP3 analysis
Lysis buffer (Dingduo, Beijing, China) was used to extract total protein from HCC, PCL, and HL tissues. Lysates were centrifuged at 11,000 g at 4 °C for 4 min. Supernatants were stored at ultra-low temperature refrigerator, and then detected in batch. Protein concentrations in the supernatants were determined by using a modified bicinchoninic acid (BCA) protein assay kit (Tiangen biomart, Beijing, China), with bovine serum albumin (BSA) as a standard.
Western blotting was performed to analysis the protein expression of GP73 and NLRP3. A total of 30 mg of total protein were separated by SDS-polyacrylamide gel electrophoresis on 4–12% Novex Bis-Tris precasting gels (Invitrogen, Carlsbad, CA, USA). Separated protein was then transferred onto nitrocellulose membranes with an iBlot Dry Blotting System (Invitrogen). Nitrocellulose membranes were blocking for 1 h in 3% TBST (BSA in Tris-buffered saline tween). After blocking, the blots were hatched with goat anti-GP73 polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) diluted at 1:800, rabbit anti-NLRP3 polyclonal antibody (Abcam, Cambridge, MA, USA) with a dilution of 1:1,000 or anti-GAPDH (glyceraldehyde phosphate dehydrogenase) antibody with a dilution of 1:1,000 (Santa Cruz Biotechnology) as a loading control. After washing three times, a 1:10,000 dilution of secondary antibodies were incubated with members. And then, the enhanced chemiluminescence (ECL) with Plus chemiluminescent detection system (Thermo, Rockford, IL, USA) was used to label the blots. Densitometric analysis of the western blotting were performed to quantify the amounts of GP73 and NLRP3 protein by using a BandScan 5.0 densitometry software, and showed as integrated intensity units relative to the GP73 and NLRP3 signal detected in healthy controls.
Follow-up of patients
Follow-up data after hepatectomy were acquired by communication with the patients or their families. Disease-free survival (DFS) and overall survival (OS) were recorded. The last follow-up date of this study was Oct. 31, 2017. One patient was lost during follow-up.
Statistical analysis
Continuous variable were described as median (range, 25th and 75th percentiles) or mean ± standard deviation (SD). Nonparametric test were used to analyze the difference of GP73 and NLRP3 protein level between HL, PCL and HCC tissue. χ2 tests were performed to investigate the correlation between GP73/NLRP3 protein expression and clinicopathologic data. Bivariate correlations were performed to evaluate the relationship between GP73/NLRP3 protein and survival time. Kaplan-Meier method and log-rank test were used to analyze OS and DFS. All tests were two-tailed and P<0.05 was considered statistically significant. Data were analyzed by SPSS 20.0 (SPSS Inc., Chicago, IL, USA).
ResultsOther Section
Patient characteristics
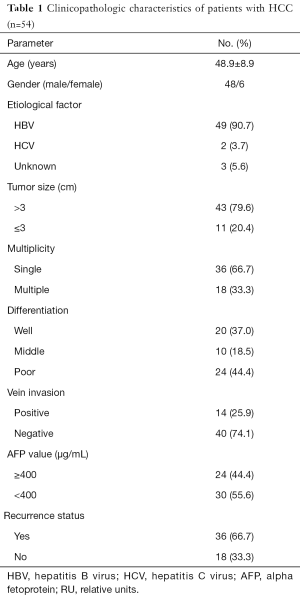
The mean age of the healthy subjects was 47.6±9.1 years with 72.7% (8/11) male. The mean age of patients with HCC was 48.9±8.9 years with 88.9% (48/54) male. As shown in Table 1, in the patients with HCC, 90.7% and 3.7% were infected with HBV and HCV, respectively. 79.6% tumor diameter was more than 3 cm and 66.7% tumor lesion was single. All the patients with HCC had different grades of cirrhosis. According to the pathological classification, 20 patients (37%) were well differentiated, 10 patients (18.5%) were middle differentiated and 24 patients (44.4%) were poorly differentiated. Vein invasion was observed in 14 patients (25.9%). AFP levels of 24 patients (44.4%) were over 400 µg/mL.
Full table
Thirty-four patients died in follow-up. Median follow-up time was 533.5 (231.3–1220.3) days. Thirty-six patients (66.7%) had recurrence during follow-up, and 26 patients relapsed within 1 year.
GP73 and NLRP3 expression in HL, PCL and HCC tissues
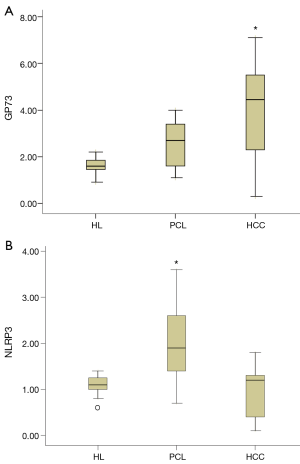
The median GP73 protein values of HL, PCL and HCC tissues were 1.6 (1.4–1.9) RU, 2.7 (1.6–3.4) RU and 4.5 (2.8–5.5) RU, respectively. As shown in Figure 1A, the GP73 protein expression in HCC tissues was significant greater than that in HL and PCL tissues (both P<0.001). The median NLRP3 protein level in HL, PCL and HCC tissues were 1.1 (0.9–1.3) RU, 1.9 (1.4–2.6) RU and 1.2 (0.4–1.3) RU, respectively. The NLRP3 level of PCL tissues were significantly higher than that of HL and HCC tissues (both P<0.001, Figure 1B).
Association between GP73/NLRP3 expression and clinicopathologic parameters
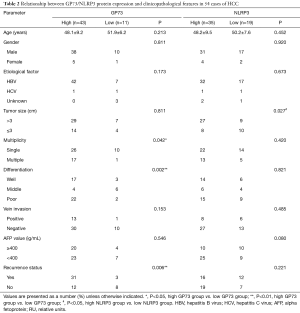
According to the GP73 level in HL and HCC tissues, 54 patients with HCC were divided into two groups: 43 patients of high GP73 expression group whose GP73 level were higher than 1.6 RU (GP73 median level in HL tissues) and 11 patients of low GP73 expression group whose GP73 level were less than 1.6 RU.
As shown in Table 2, the percentage of single tumor in low GP73 group (10/11, 90.9%) was significantly higher than that in high GP73 group (26/43, 60.5%, P<0.05). Moreover, the GP73 value of patients with single tumor were 4.2 (1.5–5.2) RU and it was significantly lower than that of patients with multiple tumor [4.8 (4.1–5.6) RU, P<0.001]. Tumor differentiation was also correlated with GP73 expression of patients with HCC. GP73 levels in poor differentiated tissues were remarkably higher than that in well differentiated tissues [4.9 (2.9–5.6) RU vs. 4.6 (3.6–5.0) RU, P<0.01]. At the same time, tumor recurrence status showed relationship with GP73 level. The GP73 expression in patients with recurrence was higher than that in patients without recurrence [4.6 (3.1–5.5) RU vs. 4.4 (1.2–5.3) RU, P<0.05].
Full table
The relationship between NLRP3 expression and clinicopathologic characteristics was investigated too. Patients with different level NLRP3 protein were divided into two groups: high NLRP3 group and low NLRP3 group. The patients in high NLRP3 group had the higher NLRP3 expression in HCC tissues than that in HL tissue. There was notably difference in tumor size between two groups. Patients with tumor diameters >3 cm had significantly less NLRP3 expression than who with tumor diameters ≤3 cm [1.1 (0.4–1.2) RU vs. 1.2 (0.8–1.3) RU, P<0.05].
Relationship between GP73 and NLRP3 expression and patient survival
The association of prognosis and GP73/NLRP3 expression was analyzed by using Bivariate Correlation. The GP73 level in HCC tissues was associated to DFS and OS, with the Pearson Correlation of −0.457 (P<0.01) and −0.357 (P<0.01). The NLRP3 level in HCC tissues was related to OS with the Pearson Correlation of 0.374 (P<0.01).
Kaplan-Meier method was used to analyze OS and DFS of patients with HCC. And the DFS and OS of 54 HCC patients were 599.1±81.1 and 905.0±98.0 days.
As shown in Figure 2A,B, survival analysis revealed that DFS and OS of patients in low GP73 group were significantly longer than that in high GP73 group (DFS: 476.8±84.1 vs. 1015.4±168.7 days, P<0.05; OS: 734.5±98.3 vs. 1346.5±109.1, P<0.01).
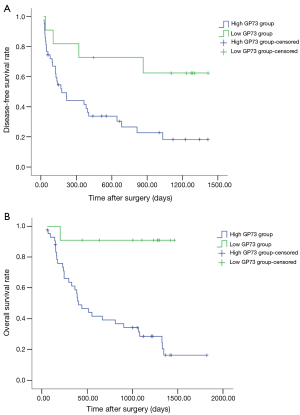
The DFS of patients in high NLRP3 group was longer than that in low NLRP3 group without significant difference (650.2±94.6 vs. 484.7±137.7, P=0.204). Meanwhile, the OS of patients in high NLRP3 group was longer than that in low NLRP3 group with significant difference (1015.8±110.1 vs. 536.1±125.0, P<0.05, Figure 3).

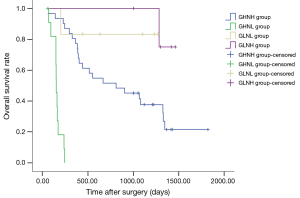
When GP73 and NLRP3 protein expressions were measured together, the patients with HCC were broken into four groups: GHNH group with high GP73 and high NLRP3 expression; GHNL group with high GP73 and low NLRP3 expression; GLNL group with low GP73 and low NLRP3 expression and GLNH group with low GP73 and high NLRP3 expression. The DFS in GLNH group (1087.8±196.1 days) was longer than that in other three groups (GHNH group: 598.8±99.5 days; GHNL: 68.0±13.2 days; GLNL group: 872.2±231.5 days). But there was no significant difference. The Kaplan-Meier survival curve of OS in four groups was shown in Figure 4. The OS of GHNH group, GHNL group, GLNL group and GLNH group were 916.8±111.3, 158.5±15.6, 1094.5±162.9 and 1416.8±38.3 days, respectively. The log-rank test showed that significant difference had existed in the four groups (P<0.001). The GLNH group had the longest OS and the GHNL group had the shortest OS.
DiscussionOther Section
HCC is one of the cancers with worst prognosis, so the early diagnosis and treatment is very important to the patients with HCC. GP73 has been known as a promising biomarker for HCC (15). It can be found in biliary epithelial cells but rarely in hepatocytes in normal human liver (15). Significantly increased expression of GP73 could be observed in viral infection, alcoholic liver disease and autoimmune hepatitis (16). Hence, the change of GP73 in liver tissues could not only for tumor progression but also for inflammatory response. In this study, GP73 level in HCC tissues was significantly higher than that in HL and PCL tissues. And it had the correlation with tumor multiplicity, differentiation and replace. The higher GP73 level means the worse tumor biology characteristic.
Inflammasomes are intracellular multiprotein complexes and play an important role for the maturation and secretion of the proinflammatory cytokines. NLRP3 inflammasome is the most extensively studied (17). It consists of a scaffold protein (NLRP3), an apoptosis-associated speck-like protein containing a caspase-recruitment domain (ASC) adaptor, and precursor pro-casp1 (18). When the body is bared to the threat of disease, the NLRP3 inflammasome takes on a guardian and is fitted out and activated to induce liver inflammatory disease and help the reconstruction of body balance. Though, chronic inflammation and inflammasome activation indicated enormous cell death, which are a cure for fibrosis, compensatory hepatic cell growth, and liver regeneration. Therefore, hepatocytes proliferating in sustained hepatic injury could amass mutations, result in abnormal hyperplasia and, finally, to tumor advance. NLRP3 protein as one of the component of inflammation was selected as our study subject. In this study, PCL tissues which were accompanied by chronic inflammation, fibrosis and cirrhosis had upregulated the expression of NLRP3 protein. Meanwhile, the expression of NLRP3 protein was notably decreased in HCC tissues, demonstrating that malignant parenchymal cells induced by the lose function of NLRP3 inflammasome. Allen et al. (19) had reported an insufficient in inflammasome was associated with cancer progression, similar prior results in colon cancer (20), which indicated that the NLRP3 inflammasome had displayed a protective effect to resist tumor progression.
In this study, we had investigated the correlation between NLRP3 expression and clinicopathologic features. The patients in high NLRP3 group had the higher NLRP3 expression in HCC tissues than that in HL tissue with significant difference. At the same time, the relationship between NLRP3 expression and patient survival was firstly revealed. The DFS of patients in high NLRP3 group was longer than that in low NLRP3 group without significant difference. But, the OS of patients in high NLRP3 group was longer than that in low NLRP3 group with significant difference. The mean OS of high NLRP3 group was 89.5% longer than that of the low NLRP3 group.
When GP73 and NLRP3 protein expressions were measured together, the patients with HCC were divided into four groups. It was interesting that the DFS and OS of the four groups were notably different. We noticed that the DFS and OS of GLNH group was longer than that in other three groups and the DFS and OS of GHNL group was the shortest among the four groups. The DFS difference had reached the astonishing 16 times between GLNH group and GHNL group. It means once upregulated GP73 and downregulated NLRP3 occurred at the same time, the patients would have a tumor recurrence in the short term. From the point of view of OS, the patients in GLNH group had about 9 times OS time, compared with the patients in GHNL group. The patients who with GP73 low expression and NLRP3 high expression would have a better prognosis. Interestingly, the DFS and OS of GLNL group were both longer than those of GHNH group. We speculated that the increase of GP73 expression may have a greater impact on tumor progression than the decrease of NLRP3 expression.
In conclusion, this is the first research of GP73 and NLRP3 protein expressions in patients with HCC. This study revealed that GP73 and NLRP3 may play the role of different level in tumor progression in patients with HCC. NLRP3 could have more effect on DFS. The mechanisms need the further study.
AcknowledgmentsOther Section
We would like to thank all the volunteers who took part in this study.
Funding: None.
FootnoteOther Section
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.04.07). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the Institutional Review Board of the Cancer Hospital of Chinese Academy of Medical Sciences (No. 17-194/1450). The clinical samples were obtained with informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Xie DY, Ren ZG, Zhou J, et al. Critical appraisal of Chinese 2017 guideline on the management of hepatocellular carcinoma. Hepatobiliary Surg Nutr 2017;6:387-96. [Crossref] [PubMed]
- Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet 2012;379:1245-55. [Crossref] [PubMed]
- Kim SH, Moon DB, Kim WJ, et al. Preoperative prognostic values of α-fetoprotein (AFP) and protein induced by vitamin K absence or antagonist-II (PIVKA-II) in patients with hepatocellular carcinoma for living donor liver transplantation. Hepatobiliary Surg Nutr 2016;5:461-9. [Crossref] [PubMed]
- Zucman-Rossi J, Villanueva A, Nault JC, et al. Genetic Landscape and Biomarkers of Hepatocellular Carcinoma. Gastroenterology 2015;149:1226-39.e4. [Crossref] [PubMed]
- Bruix J, Sherman MPractice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology 2005;42:1208-36. [Crossref] [PubMed]
- Kladney RD, Bulla GA, Guo L, et al. GP73, a novel Golgi-localized protein upregulated by viral infection. Gene 2000;249:53-65. [Crossref] [PubMed]
- Mao Y, Yang H, Xu H, et al. Golgi protein 73 (GOLPH2) is a valuable serum marker for hepatocellular carcinoma. Gut 2010;59:1687-93. [Crossref] [PubMed]
- Chen X, Wang Y, Tao J, et al. mTORC1 Up-Regulates GP73 to Promote Proliferation and Migration of Hepatocellular Carcinoma Cells and Growth of Xenograft Tumors in Mice. Gastroenterology 2015;149:741-52.e14. [Crossref] [PubMed]
- Yang H, Xu H, Wang Y, et al. Clinicopathologic correlations of Golgi protein 73 and signal transducer and activator of transcription 3 expression in hepatocellular carcinoma. Transl Cancer Res 2017;6:238-46. [Crossref]
- Strowig T, Henao-Mejia J, Elinav E, et al. Inflammasomes in health and disease. Nature 2012;481:278-86. [Crossref] [PubMed]
- Zaki MH, Boyd KL, Vogel P, et al. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity 2010;32:379-91. [Crossref] [PubMed]
- Franchi L, Eigenbrod T, Muñoz-Planillo R, et al. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol 2009;10:241-7. [Crossref] [PubMed]
- Sun Y, Yang H, Mao Y, et al. Increased Golgi protein 73 expression in hepatocellular carcinoma tissue correlates with tumor aggression but not survival. J Gastroenterol Hepatol 2011;26:1207-12. [Crossref] [PubMed]
- Fimmel CJ, Wright L. Golgi protein 73 as a biomarker of hepatocellular cancer: development of a quantitative serum assay and expression studies in hepatic and extrahepatic malignancies. Hepatology 2009;49:1421-3. Erratum in: Hepatology 2009;50:331. [Crossref] [PubMed]
- Kladney RD, Cui X, Bulla GA, et al. Expression of GP73, a resident Golgi membrane protein, in viral and nonviral liver disease. Hepatology 2002;35:1431-40. [Crossref] [PubMed]
- Ganz M, Csak T, Nath B, et al. Lipopolysaccharide induces and activates the Nalp3 inflammasome in the liver. World J Gastroenterol 2011;17:4772-8. [Crossref] [PubMed]
- Church LD, Cook GP, McDermott MF. Primer: inflammasomes and interleukin 1beta in inflammatory disorders. Nat Clin Pract Rheumatol 2008;4:34-42. [Crossref] [PubMed]
- Allen IC, TeKippe EM, Woodford RM, et al. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J Exp Med 2010;207:1045-56. [Crossref] [PubMed]
- Zaki MH, Vogel P, Body-Malapel M, et al. IL-18 production downstream of the Nlrp3 inflammasome confers protection against colorectal tumor formation. J Immunol 2010;185:4912-20. [Crossref] [PubMed]


