
Synchronous organizing pneumonia after sequential three-dimensional radiotherapy for three lung metastases from hepatocellular carcinoma: a case report
Introduction
Radiation-induced lung injury has been reported after postoperative radiotherapy for breast cancer (1-4); currently, it is a well-known complication of thoracic radiation for lung, esophageal, and hematologic malignancies. The incidence of radiation-induced pneumonitis, mostly of grade 2 or less, ranges from approximately 10% to 30%, and it is manageable (5,6). Radiation-induced lung injury can be categorized into radiation pneumonitis (RP) and organizing pneumonia (OP) (7). OP included RP in the broad sense, but since Crestani et al. (7) reported OP in 1998, several authors have described the features of OP, which differ from those of RP (8,9). Differences between RP and OP have been reported in terms of the time to appearance, predictive factors, expression mechanism, and migration of lung infiltration (1-3). However, to the best of our best knowledge, no author has demonstrated the time to OP appearance after sequential radiotherapy for ipsilateral lung tumors in the second radiotherapy cycle same patient. In this rare case, a woman in her 70s with three lung metastases developed OP synchronously in each irradiated area after sequential three-dimensional radiotherapy (3D-CRT).
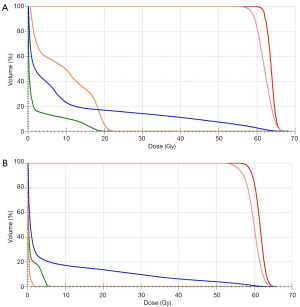
Case presentation
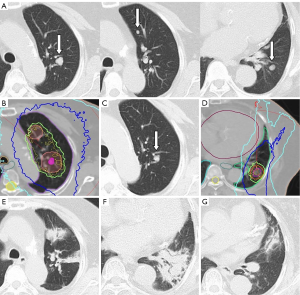
We report the case of a woman in her 70s who had three metastases in the left lung from hepatocellular carcinoma (HCC). She was a lifelong non-smoker and had no significant past history of collagen vascular diseases, except diabetes. Her general condition was good, and she had no respiratory symptoms (performance status 1). Transarterial chemoembolization (TACE) was conducted for HCC before lung metastases developed, and her liver status was stable, with no new lesions at any other organs, except the left lung. There were three nodules in the upper (two lesions) and lower (one lesion) lobes of the left lung, which gradually increased in size (Figure 1A). Prothrombin induced by vitamin K absence-II levels also increased to >1,000 mAU/mL, but no other metastases were detected on computed tomography (CT). Therefore, we judged that these lung nodules were metastases from HCC. She was recommended to use sorafenib for lung metastases, but she refused due to side effects and chose to undergo 3D-CRT. The degree of Krebs von den Lungen-6 (KL-6) was within normal limits at this time. Her respiratory function was low (vital capacity: 1.37 L, 1-s forced expiratory volume: 0.95 L), but she could endure 3D-CRT. We decided to sequentially administer 3D-CRT at a total dose of 60 Gy in 15 fractions as an alternative to stereotactic body radiotherapy for lung metastases after considering the side effects, patient’s age (relatively old), number of metastases, and respiratory function. The treatment plan was implemented using CT with a long scan time (3 s) under resting respiratory conditions. Scans were assessed in 3-mm sections at the lesion site and 5-mm sections elsewhere. Because a long scan time was used under resting respiration, the clinical target volume (CTV) was used to define the visible range of CT. The internal target volume (ITV) is the CTV plus the tumor margins for organ movement. ITV included a 5-mm “set up” margin to establish the planning target volume (PTV). The radiation field was defined as PTV plus a 5-mm leaf margin. The two upper lobe lesions were closely located and were therefore irradiated together in one field. Using 6-MV photons, 3D-CRT (all coplanar irradiation) was administered under resting respiration (Figure 1B). Three months after the end of 3D-CRT, lung metastases of the upper lobe exhibited a good response and decreased in size, and there were no changes outside or inside the radiation field on chest radiographs and CT (Figure 1C). KL-6 was also maintained within normal limits. We decided to administer 3D-CRT for a second time due to metastasis to the lower lobe of the lung (Figure 1D). The 3D-CRT method and radiation dose were similar to those used for the first treatment, but the irradiated area was not duplicated. At 1.5 months after the end of the second 3D-CRT course, the patient presented with fatigue, dry cough, shortness of breath, and dyspnea; at 2 months from the second radiotherapy cycle, dense pneumonic infiltrates with air bronchograms within the first and second radiation fields and outside the field were synchronously detected on CT images (Figure 1E,F,G). The V20 (lung volume covered by ≥20 Gy) was reduced to a safe range: 16.9% at the first therapy and 13.7% at the second therapy (Figure 2A,B). Clinical examination revealed crackles and bronchial breath sounds. Peripheral O2 saturation was 93% on room air, as measured by a pulse oximeter. Blood analysis revealed a C-reactive protein level of 11.95 mg/L, without clinical signs of viral or bacterial infections in any other organ; furthermore, the white blood cell count was 4,700/µL, without the elevation of eosinophil counts. KL-6 was increased to >1,000 U/mL. Additional serum chemistry examinations, renal and liver function tests, and urinalysis revealed no changes. No other treatments such as TACE and oral chemotherapy were conducted in this period. A clinical diagnosis of OP grade 2 (symptomatic but does not require O2 supplementation) was made according to the Common Terminology Criteria for Adverse Events 4.0. We recommended steroid administration, but the patient did not agree due to concerns regarding blood sugar elevation. She was only observed carefully, and an improvement in the clinical symptoms naturally occurred within 28 days. Chest radiograph showed improvement of the consolidation. A diagnosis of lung injury, most probably OP, due to radiotherapy was made.
Discussion
Several reports have described the interval from radiotherapy for lung tumors to the development of RP and OP, but no reports have mentioned the time to OP appearance after sequential 3D-CRT for ipsilateral lung tumors in the same patient. This is the first case in which RP synchronously occurred after 3D-CRT conducted at different times.
Radiation-induced lung injury can be divided into two types: RP and OP (1,2,7,10). Several differences between RP and OP have been reported. RP is associated with direct damage and interstitial pulmonary inflammation with an alveolar exudative component (11), whereas an autoimmune mechanism is assumed to be involved in the occurrence of OP (12). RP lesions are characterized by alveolar consolidation limited to the irradiation field, whereas OP lesions are located outside the irradiation field and frequently migrate to other areas or even the opposite lung (1,2,13). RP can progress to irreversible lung injury and develop into radiation fibrosis, whereas OP usually disappears without fibrosis, but occasionally relapses when the corticosteroid dosage is decreased (1,2). RP occurs in almost all patients who have received radiotherapy, whereas the frequency of OP is low. We considered that the present case most probably fit the definition for OP because of the exclusive radiotherapy, time to appearance, location of the infiltration inside/outside the irradiation field, symptoms, clinical progression, and contradictory eosinophilic and infection pneumonia.
The time to appearance of lung injury after irradiation has also been previously reported. RP arises during radiotherapy or shortly after radiotherapy, whereas OP tends to arise several months to within 1 year after the completion of radiotherapy, which is generally longer than the time to RP (1,2,9). In particular, the interval from radiotherapy for lung cancer to the development of OP ranged from 6 to 18 months (3,4,10). In our case, the time to appearance after the second radiotherapy cycle was shorter than that described in these reports. OP occurred at 5.5 months after the first 3D-CRT course but only 1.5 months after the second 3D-CRT course and synchronously occurred in the first and second irradiated areas. Several authors have reported that radiation-induced pneumonitis has been related to an immunologically mediated mechanism, i.e., a form of lymphocyte-mediated hypersensitivity reaction with the elevation of neutrophils, mast cells, and eosinophils and an increase in the CD4/CD8 ratio detected on bronchioalveolar lavage (BAL) (14,15). OP development may involve the T-cell and Fas/Fas-ligand pathways, which can be activated by lung irradiation (16,17). Martin et al. (18) also reported that lung irradiation induced the elevation of lymphocytes in not only the irradiated area but also outside the irradiated area. According to these reports, we considered that the first 3D-CRT course for upper-lobe metastasis stimulated the immunologically mediated mechanisms in the entire left lung and maybe also the right lung without radiological findings, and the second 3D-CRT course further activated the immunologically mediated mechanism to induce the occurrence of OP within a short time in both the first and second irradiated areas and outside areas. In this case, in which sequential radiotherapy was conducted, OP occurred within a shorter time than that previously reported.
Some investigations have demonstrated that predictive factors related to the patients such as age, pulmonary emphysema, PO2 levels, and pulmonary function (forced vital capacity) and related to the radiotherapy regimen such as V20 and mean lung dose (MLD) are associated with the development of RP. RP itself may be related to OP (1,6,19). Although our patient was in her 70s, radiotherapy for elderly patients is widely conducted. Additionally, although the respiratory function seemed to be low, she was generally able to endure 3D-CRT. Therefore, there were no particular problems related to patient factors. Regarding the 3D-CRT regimen, we made an effort to reduce the irradiated lung volume to the required value; however, a radiation dose of <0.5 Gy is sufficient to induce a bystander effect (20). We should be careful while treating multiple fields or using intensity-modulated radiotherapy even if the V20 and MLD are within normal ranges because low doses can spread widely in the lung field, especially the added doses of sequential 3D-CRT.
The retrospective nature of the study and the lack of immunological and histological evaluation using BAL or pathological findings obtained via open/video-assisted thoracoscopy or transbronchial biopsy pose limitations. The diagnosis of OP was determined based only on the clinical course and radiological findings, because our patient was elderly and the symptoms were too mild for her to undergo invasive examinations. Further investigations are needed to resolve such limitations.
Conclusions
This case report draws attention to the development of synchronous OP after sequential radiotherapy and advises careful follow-up with the initiation of a second radiotherapy cycle even if OP does not appear for several months after the first radiotherapy cycle.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.03.11). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Oie Y, Saito Y, Kato M, et al. Relationship between radiation pneumonitis and organizing pneumonia after radiotherapy for breast cancer. Radiat Oncol 2013;8:56. [Crossref] [PubMed]
- Epler GR, Kelly EM. Systematic review of postradiotherapy bronchiolitis obliterans organizing pneumonia in women with breast cancer. Oncologist 2014;19:1216-26. [Crossref] [PubMed]
- Murai T, Shibamoto Y, Nishiyama T, et al. Organizing pneumonia after stereotactic ablative radiotherapy of the lung. Radiat Oncol 2012;7:123. [Crossref] [PubMed]
- Takeda A, Oku Y, Sanuki N, et al. Feasibility study of stereotactic body radiotherapy for peripheral lung tumors with a maximum dose of 100 Gy in five fractions and a heterogeneous dose distribution in the planning target volume. J Radiat Res 2014;55:988-95. [Crossref] [PubMed]
- Matsuo Y, Shibuya K, Nakamura M, et al. Dose-volume metrics associated with radiation pneumonitis after stereotactic body radiation therapy for lung cancer. Int J Radiat Oncol Biol Phys 2012;83:e545-9. [Crossref] [PubMed]
- Kim K, Lee J, Cho Y, et al. Predictive factors of symptomatic radiation pneumonitis in primary and metastatic lung tumors treated with stereotactic ablative body radiotherapy. Radiat Oncol J 2017;35:163-71. [Crossref] [PubMed]
- Crestani B, Valeyre D, Roden S, et al. Bronchiolitis obliterans organizing pneumonia syndrome primed by radiation therapy to the breast. The Groupe d’Etudes et de Recherche sur les Maladies Orphelines Pulmonaires (GERM“O”P). Am J Respir Crit Care Med 1998;158:1929-35. [Crossref] [PubMed]
- Gudavalli R, Diaz-Guzman E, Arrossi AV, et al. Fleeting alveolar infiltrates and reversed halo sign in patients with breast cancer treated with tangential beam irradiation. Chest 2011;139:454-9. [Crossref] [PubMed]
- Ogo E, Komaki R, Fujimoto K, et al. A survey of radiation-induced bronchiolitis obliterans organizing pneumonia syndrome after breastconserving therapy in Japan. Int J Radiat Oncol Biol Phys 2008;71:123-31. [Crossref] [PubMed]
- Ochiai S, Nomoto Y, Yamashita Y, et al. Radiation-induced organizing pneumonia after stereotactic body radiotherapy for lung tumor. J Radiat Res 2015;56:904-11. [Crossref] [PubMed]
- Satoh K, Kobayashi T, Misao T, et al. CT assessment of subtypes of pulmonary emphysema in smokers. Chest 2001;120:725-9. [Crossref] [PubMed]
- Miwa S, Morita S, Suda T, et al. The incidence and clinical characteristics of bronchiolitis obliterans organizing pneumonia syndrome after radiation therapy for breast cancer. Sarcoidosis Vasc Diffuse Lung Dis 2004;21:212-8. [PubMed]
- Izumi T, Kitaichi M, Nishimura K, et al. Bronchiolitis obliterans organizing pneumonia: Clinical features and differential diagnosis. Chest 1992;102:715-9. [Crossref] [PubMed]
- Majori M, Poletti V, Curti A, et al. Bronchoalveolar lavage in bronchiolitis obliterans organizing pneumonia primed by radiation therapy to the breast. J Allergy Clin Immunol 2000;105:239-44. [Crossref] [PubMed]
- Roberts CM, Foulcher E, Zaunders JJ, et al. Radiation pneumonitis: a possible lymphocyte mediated hypersensitivity reaction. Ann Intern Med 1993;118:696-700. [Crossref] [PubMed]
- Chakraborty M, Abrams SI, Camphausen K, et al. Irradiation of tumor cells up-regulates Fas and enhances CTL lytic activity and CTL adoptive immunotherapy. J Immunol 2003;170:6338-47. [Crossref] [PubMed]
- Garnett CT, Palena C, Chakraborty M, et al. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res 2004;64:7985-94. [Crossref] [PubMed]
- Martín C, Romero S, Sánchez-Payá J, et al. Bilateral lymphocytic alveolitis: a common reaction after unilateral thoracic irradiation. Eur Respir J 1999;13:727-32. [Crossref] [PubMed]
- Kocak Z, Borst GR, Zeng J, et al. Prospective assessment of dosimetric/physiologic-based models for predicting radiation pneumonitis. Int J Radiat Oncol Biol Phys 2007;67:178-86. [Crossref] [PubMed]
- Matsumoto H, Tomita M, Otsuka K, et al. A new paradigm in radioadaptive response developing from microbeam research. J Radiat Res 2009;50 Suppl A:A67-79.


