
Dosimetric characteristics of INTRABEAM® flat and surface applicators
Introduction
The Zeiss INTRABEAM® Radiotherapy System (IRS) is a 50 kV X-ray unit that is designed for interstitial brachytherapy. This novel technology was originally the Photon Radiosurgery System (PRS) developed by the Photoelectron Corporation in Massachusetts, USA and is now commercially available as the Zeiss IRS.
The devise has been in use for IORT with the spherical applicators. The dosimetric characteristics of the PRS source and those of the spherical applicators have been well studied and reported (1-7). The clinical efficacy and results are released in the TARGIT-A Trials reports (8,9).
With appropriate collimation and differential attenuation, the PRS source has been modified to direct the beam as a forward projection radiation source for skin cancer treatment (10). Further refinement of the applicators by Carl Zeiss Meditec Inc. made available two sets of forward beam projecting applicators: INTRABEAM® Flat applicator (IFA) and the INTRABEAM® Surface applicator (ISA). The manufacturer submitted the applicators, which are classified as medical device, to the FDA for approval on 3/4/2013 and it was deemed Substantially Equivalent on 6/26/2013.
Materials and methods
Gafchromic EBT 2 QD+ (GEBT) film was used together with the solid water phantom for capturing the integrated exposure from each of the applicators in axial and cross-section of the beam. The GEBT film was selected due to the convenience and the reported relative independence of energy at the 50 kV range (11-14).
In order to eliminate the possible concern of sensitivity to energy changes due to filtration and the dose rate on film sensitivity the Hurter-Drifield sensitometric curve (H-D) was established by direct relationship of ionization chamber measurements using PTW Unidose electrometer and IC ionization chamber type 34013 in the INTRABEAM® water phantom system with the optical densities measured by the Vidar Dosimetry Pro Advantage® film scanner from the GEBT film. The points of measurement were at the exact corresponding positions relative to the surface of the applicators (Figure 1, Figure 1A-C). A polynomial expression was fitted to the ion chamber measurements and the optical density for each of the applicator sets as an analogy relation (Figure 1D, E) for 2D dosimetry analysis.
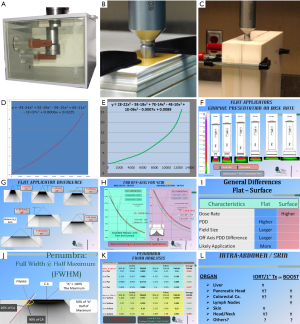
Imaging processing and isodose generation was performed using the Radiological Imaging Technology® software with special attention to the film H-D calibration.
Dosimetric characteristics
Ion chamber and film data were analysed for both conventional characteristics and unique characteristics of these short source-to-skin distance applicators. Analysis was performed on the conventional dosimetric characteristics included Dose Rate (DR) and Central Axis Percentage Depth Dose (PDD).
On account of the uniqueness of the 50 kV beam energy, together with the short target-to-surface distance (TSD) and the individualized filtration for the uniform isodose curve at a 5 mm tissue depth for the IFA and uniform isodose curve at the surface for the ISA; analysis was considered for the unconventional dosimetric characteristics. The investigation included Beam Divergence, Beam Hardening effects of off-central-axis Beam Quality expressed in PDD and the Penumbra width of the applicators which are expressed in the full-width-half-maximum index (FWHM) for the measurement for different sizes.
Evaluation of the two sets of applicators, gave us an appreciation on the characteristic differences between the IFA and the ISA. Consultations with physicians gave us some insight on the potential clinical applications for these forward projecting applicators.
Results
Conventional dosimetry characteristics
Figure 1F,G and Figure 2, Figure 2A,B represent a graphic presentation (not to scale) of two dimensional views of both dose rate and percentage depth dose relationships as a function of applicator type and cone size.
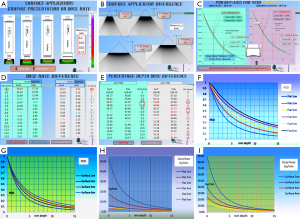
Output-factors
Contrary to conventional wisdom in radiotherapy that larger field sizes have larger dose rate, these applicators have the highest dose rate with the smallest field size applicator (Figure 2H,I).
Flat applicators have a lower dose rate compared to those of the Surface applicators of the same size (Figure 2D) (note: unit of the table is charge per unit time)
Surface applicators are two to three times higher in dose rate than those of the Flat applicators of a given depth of the same size.
Percentage depth dose
PDD’s increases with field size of the same type of applicator; Flat applicators are about 1.5 time higher in PDD value than the Surface applicator of the same size at a given depth, on account more attenuation by the flattening filter (Figure 2E-G).
Unconventional dosimetry characteristics
Beam divergence
The virtual-source-to-surface distance of the applicator distance (indicated as Y in Figure 1G) (Figure 2B), and the divergent angle indicated as angle (Ɵ) increases as field size increases.
Flat applicators (Figure 1G )
The (Y mm, Ɵ°) values of 1, 2, 3, 4, 5, 6 cm diameter cones are determined graphically. The values are (14.5, 55°), (19.0, 65°), (23.0, 75°), (26.5,82°), (29.3, 88°) and (30.5, 94°) respectively.
Surface applicators (Figure 2B )
The (Y mm, Ɵ°) values of 1, 2, 3, 4, cm diameter cones are (9.5, 55°), (14.0, 68°), (18.0, 78°), (21.5, 84°) respectively.
Between the Flat and Surface applicator sets of the same size, the divergent angles are very similar; whereas the ISA have an approximately 5.0 mm shorter virtual-source-to-surface. Together with less attenuation for the ISA as compared to the IFA, it explains its higher dose rate as compared to the ISA with identical kV and mA, as compared to those of the IFA of the same size.
Off-central-axis beam quality
The largest ISA is a 4.0 cm diameter (Figure 2C), for comparison same size IFA were studied (Figure 1H, Figure 2C). There is more attenuation for the IFA. The Dose rate at depth was measured at the central axis, as well at 7.0 mm and at 14.0 mm off central axis. PDD was determined by normalizing each set of data to the maximum dose rate for each off-axis distance.
Flat applicators
Figure 1H demonstrated that the dose rate of the three axes are within ±2% throughout different depths. The PDD of the central axis shows a higher PDD at any given depth. At 5 mm depth, the ratio of PDD of central, 7, 14 mm are 1.0, 0.973 and 0.922. The farther off axis beams are of softer X-rays.
Surface applicators
Figure 2C demonstrated that the dose rate of three axes are within ±4% throughout different depths. The PDD of the central axis shows less differences in PDD at any given depth. At 5 mm depth, the ratio of PDD of central, 7, 14 mm are 1.0, 0.997 and 0.970. The off axis beams are of rather similar X-rays quality for the ISA.
Penumbra (FWHM)
Figure 1J,K illustrates the FWHM index and the values measured for both IFA and ISA. For comparison purposes, only cone sizes of 1, 2, 3 and 4 cm were measured. In general the penumbra size was expressed as distance between the 90% and the 20% divergent lines of central axis maximum value. The result is around 1% of the field width. There is not much of difference between the two types of applicators.
Conclusions and discussion
This study using an ionization chamber for calibrated dose rate measurements and GEBT film for 2D analysis, gave a general understanding of the basic characteristics of the two sets of forward beam applicators. Figure 1I listed the general differences between the applicators sets.
On the whole the IFA set provided a larger field size, and higher PDD and it may indicate a wider clinical application eventually. Whereas at the lower dose rate, it was two to three times lower as compared to the ISA. The different quality of radiation across the larger field sizes may raise possible concern for relative biological effectiveness (RBE) differences within a treatment field. These factors may shift favor to the Surface applicators.
General awareness on the differences between the two sets of applicators should be considered in evaluating clinical application and analyzing the clinical results.
In consultation with clinical colleagues, Figure 1L summarizes our consideration in using the applicators for intra-pelvic, intra-abdominal and skin applications.
Reviewing the recent publication by Schneider F et al. (15) in January 2014 on their investigation of the flat and surface applicators and a case report of using a Flat applicator, we concur with their findings that the flat applicators have better dose homogeneity across beam profile, and surface applicators show a steeper depth dose gradient and higher dose rate.
In the USA, clinical approval for patient treatment with the flat applicators only happened in the beginning of Feb. 2014. We did however treat one patient with a 3.5 cm spherical applicator for gastrointestinal recurrent cancer by individualized shielding recently.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Frederik Wenz and Elena Sperk) for the series “Intraoperative Radiotherapy” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2014.02.02). The series “Intraoperative Radiotherapy” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Biggs PJ, Beatty J, Yasuda T. Distance-dose curve for a miniature x-ray tube for stereotactic radiosurgery using an optimized aperture with a parallel-plate ionization chamber. Med Phys 1999;26:2550-4. [PubMed]
- Kraus-Tiefenbacher U, Steil V, Bauer L, et al. A novel mobile device for intraoperative radiotherapy (IORT). Onkologie 2003;26:596-8. [PubMed]
- Yasuda T, Beatty J, Biggs PJ, et al. Two-dimensional dose distribution of a miniature x-ray device for stereotactic radiosurgery. Med Phys 1998;25:1212-6. [PubMed]
- Armoogum KS, Parry JM, Souliman SK, et al. Functional intercomparison of intraoperative radiotherapy equipment - Photon Radiosurgery System. Radiat Oncol 2007;2:11. [PubMed]
- Schneider F, Fuchs H, Lorenz F, et al. A novel device for intravaginal electronic brachytherapy. Int J Radiat Oncol Biol Phys 2009;74:1298-305. [PubMed]
- Ebert MA, Carruthers B. Dosimetric characteristics of a low-kV intra-operative x-ray source: implications for use in a clinical trial for treatment of low-risk breast cancer. Med Phys 2003;30:2424-31. [PubMed]
- Eaton DJ. Quality assurance and independent dosimetry for an intraoperative x-ray device. Med Phys 2012;39:6908-20. [PubMed]
- Vaidya JS, Baum M, Tobias JS, et al. Targeted intra-operative radiotherapy (Targit): an innovative method of treatment for early breast cancer. Ann Oncol 2001;12:1075-80. [PubMed]
- Vaidya JS, Wenz F, Bulsara M, et al. Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT-A randomised trial. Lancet 2014;383:603-13. [PubMed]
- Bodner WR, Hilaris BS, Alagheband M, et al. Use of low-energy X-rays in the treatment of superficial nonmelanomatous skin cancers. Cancer Invest 2003;21:355-62. [PubMed]
- Rampado O, Garelli E, Deagostini S, et al. Dose and energy dependence of response of Gafchromic XR-QA film for kilovoltage x-ray beams. Phys Med Biol 2006;51:2871-81. [PubMed]
- Cheung T, Butson MJ, Yu PK. Independence of calibration curves for EBT Gafchromic films of the size of high-energy X-ray fields. Appl Radiat Isot 2006;64:1027-30. [PubMed]
- Butson MJ, Cheung T, Yu PK. Weak energy dependence of EBT gafchromic film dose response in the 50 kVp-10 MVp X-ray range. Appl Radiat Isot 2006;64:60-2. [PubMed]
- Ebert MA, Asad AH, Siddiqui SA. Suitability of radiochromic films for dosimetry of very-low energy X-rays. J Appl Clin Med Phys 2009;10:2957. [PubMed]
- Schneider F, Clausen S, Thölking J, et al. A novel approach for superficial intraoperative radiotherapy (IORT) using a 50 kV X-ray source: a technical and case report. J Appl Clin Med Phys 2014;15:4502. [PubMed]


