
Prognostic factors for unresectable hepatocellular carcinoma treated with transcatheter arterial chemoembolisation combined with radiotherapy
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the second leading cause of cancer-related death worldwide (1). Due to the characteristics of intrahepatic metastasis and the aggressiveness of the disease, the prognosis is very poor. Surgical resection is the preferred method for the treatment of HCC, however 80% of patients have lost the chance of surgery because of poor liver function and advanced tumor stage (2). For these unresectable HCC (uHCC), transcatheter arterial chemoembolisation (TACE) is the most commonly used treatment. However, previous studies have shown that the tumor necrosis rate was less than 44% when the tumor diameter greater than 3 cm and the recurrence and metastasis of the tumor would increase when incomplete tumor necrosis was triggered (3,4). It is difficult to achieve satisfactory results only by TACE, therefore, other additional treatment strategies must be added for the treatment of this disease. As the whole liver has a low radiation tolerance, the role of radiotherapy (RT) is limited in the treatment of HCC (5). The development of three-dimensional conformal radiotherapy (3D-CRT) and intensity-modulated radiation therapy (IMRT) make it possible to achieve the radical tumor dose by irradiating tumor tissue accurately and reduce the irradiation dose of surrounding normal organs and the remaining normal liver tissue. RT has been proven to be adaptable for all stages of HCC (6) and has been incorporated into the comprehensive treatment of liver cancer in the National Comprehensive Cancer Network (NCCN) guidelines of 2015 (NCCN guidelines Version 1.2015).
Many studies have shown that TACE combined with RT was more advantageous than TACE alone for uHCC patients in terms of the short-term efficacy or long-term survival (7-11). The objective response varied widely from 18.0% to 90.5% and the 2-year overall rate ranged from 38% to 61.3% (7,8,11). Therefore, it is significant to determine the sensitivity factors of the disease, as well as the treatment sensitivity before commencing treatment.
The independent factors that influence prognosis are still not clear. We analysed the clinical data of uHCC patients treated with TACE combined with RT in Shandong Cancer Hospital affiliated to Shandong University from January 2010 to December 2015 in this study. This study was conducted to determine the factors affecting the survival of patients with uHCC treated with TACE plus RT and provide individualised treatment for uHCC patients.
Methods
Patients
Patients who met the study criteria in Shandong Cancer Hospital affiliated to Shandong University were fully evaluated. The eligibility criteria were as follows: (I) diagnosed by the European Association for the Study of the Liver (EASL) criteria (12); (II) did not received prior treatment; (III) Karnofsky performance score (KPS) ≥70; (IV) Child-Pugh class A or B; (V) treated with TACE plus RT and (VI) complete follow-up was available. The exclusion criteria included hepatic arteriovenous fistula, patients with severe liver dysfunction caused by cirrhosis, and other serious systemic diseases (respiratory system, digestive system, endocrine system and cardiovascular system diseases), which may seriously affect the prognosis of HCC. All analyses were performed in compliance with the Ethical Principles for Medical Research Involving Human Subjects outlined in the Helsinki Declaration in 1975 (revised in 2000). The study protocol was approved by the Institutional Ethics Committee of the Shandong Cancer Hospital. Informed consent was obtained from all included patients.
Treatment
The interval between TACE was 4–6 weeks, and RT was performed at 4 weeks after TACE.
TACE
Following preoperative examinations, and after exclusion of taboos, patients maintained the supine position on the treatment bed. The right inguinal ligament at the bottom of the middle point 2 cm was selected for local anesthesia, subsequently, the Seldinger method was used to insert the catheter into the hepatic artery or superior mesenteric artery via the femoral artery. The end-point of TACE was apparent when there is stagnation of blood flow in the artery supplying the tumor or a complete uptake of lipiodol within the tumor. Subsequently, 750–1,000 mg of 5 fluorouracil (5-Fu), 100–150 mg of oxaliplatin (L-OHP), 10–30 mg of hydroxycamptothecin (HCPT) and 5–10 mg of mitomycin (MMC) were selectively injected according to the size and blood supply of the liver tumor, followed by lipiodol and gelatin sponge, lipiodol was used as an emulsion. As such, 59 patients were selected for 5-Fu, HCPT, and MMC; 26 patients for HCPT and MMC; and 16 patients for L-OHP and MMC. The puncture point was bandaged after the operation, and the lower extremities were stable for 12 hours. The liver function was evaluated after TACE, and TACE time ranged from 1 to 12 times depending on the patient's condition and tolerance, TACE time was from 1 to 12.
RT
For simulations, patients were in the supine position in the treatment bed with their arms fixed on their heads to determine the position using vacuum casts. Additionally, patients’ abdomens were compressed to reduce the uncertainty bias caused by organ motion and respiratory movements. The laser line location was used to mark the relative position of the treatment bed, the negative pressure zone and the patient’s body surface. Approximately, 10–20 minutes before the scan, 150–300 mL of oral contrast medium was used, and scanning was performed from the top of diaphragm to the inferior border of liver by 3 mm thickness. All plans were designed on the Varian Eclipse version 8.6.23 treatment planning system. Gross tumor volume (GTV) was identified combining with TACE after lipiodol deposition, including portal vein thrombosis. Clinical target volume was GTV plus 0.5–1 cm. Planned target volume was defined as CTV plus 0.5 cm in the left and right direction, but plus 1.5–2 cm in the upward and downward directions to address the error of respiratory organ motion. Subsequently, the physician made an RT plan, with two radiologists and physicists assessing whether the clinical requirements were met and the dose validated. In this study, 80 patients were treated with 3D-CRT and 21 patients were IMRT. Conventional fractionation irradiation was performed (1.8–2.0 Gy daily, 5 times a week, fractionation irradiation was performed in 1 to 2 months). The irradiation dose and time were determined by the characteristic of tumor and normal adjacent tissues.
Evaluation
Abdominal computed tomography (CT) was performed to evaluate the treatment efficacy every 3 months in the first year following treatment, then half year a time after one year. Tumor response was assessed based on the modified Response Evaluation Criteria in Solid Tumors Version 1.1 (mRECIST 1.1) evaluation criteria: complete response (CR), where the tumor markers (AFP) are in the reference range and complete disappearance of all target lesions, or all of the target lesions disappeared in the arterial phase, and no new lesions appeared, and this was maintained over 4 weeks periods. Partial response (PR) was defined as a decrease of >30% in total reduction of baseline lesion diameter (enhanced development of arterial phase). Progressive disease (PR) was defined as an increase of >20% in the sum of baseline lesions diameter (enhanced development of arterial phase) or new lesions. Between CR and PR was stable disease (SD) (13). CR and PR were regarded as object response.
Toxicities were graded using the National Cancer Institute Common Toxicity Criteria Version 3.0 (NCI-CTCAE v3.0).
Statistical analysis
The survival time was calculated from the time of beginning of treatment by the Kaplan-Meier method. To identify the prognostic factors for survival, univariate and multivariate analyses by Cox proportional regression model using forward LR stepwise regression were performed. All the statistical analyses were performed using SPSS 19.0 (IBM Corporation, Armonk, NY, USA). A value of <0.05 was considered statistically significant.
Results
Patient characteristics and treatment
All patient data are shown in Table 1. The median age of patients was 59 years (range, 29–78 years). Portal vein thrombus (PVT) of type I was seen in 10 patients, type II in 12 patients and type III in 4 patients. The median diameter was 5.0 cm (0.6–17 cm). The median number of TACE was 2 (range, 1–12) with intervals of 4–6 weeks if it produced a response. All patients used the conventional fractionation RT with a total dose of 50.35±11.53 Gy and median dose of 52.00 Gy. The median volume of GTV was 111.30 cm3 (range, 3.51–1,597.60 cm3). Lung metastases present was found in 1 patient at diagnosis. During follow-up, 21 patients had metastases, 6 had lung metastases, 8 had intrahepatic metastases, 3 had bone metastases, 2 had kidney metastases and 2 bad both lung and intrahepatic metastases. For patients with progress or metastases, additional therapy was required after their initial treatment, including RT (location of metastasis and recrudescence) and chemotherapy. These patients were not excluded.
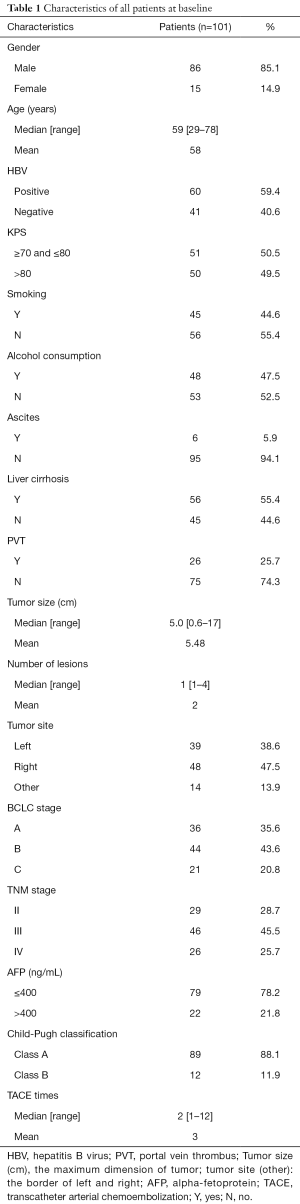
Full table
Response and survival
The curative effect was evaluated 4–6 weeks after the end of the treatment, the objective response rate was 73.3% with 4 patients (4.0%) in CR and 70 patients (69.3%) in PR. Stable disease was obtained in 17 patients (16.8%) and progressive disease in 10 patients (9.9%). After adjustment for potential confounders, the TNM stage (P=0.008), Child-Pugh classification (P=0.006) and the total liver volume receiving >20 Gy (V20) (P=0.046) were independent prognostic factors for tumor response.
The median follow-up was 28 months (range, 5–60 months) after the initiation of treatment. The median survival time was 23 months (range, 3–60 months). At the time of analysis, 75 patients had died and 26 patients were still alive. The overall survival rates at 1-, 3-, and 5-year were 80.2%, 26.7% and 7.9%, respectively (median survival 23 months).
Toxicity
Nausea and vomiting were notable in 20 patients with grade I, 12 with grade II, but it was self-limiting in all patients. Fever occurred frequently in 2 of 5 patients, but soon subsided without any treatment. Grade I of leukopenia of was seen in 32 patients (31.7%), grade II in 12 (12.0%) and grade III in 3 (3.0%), but all patients eventually recovered after accepting some treatment. Aminotransferase was elevated in 21 patients, and bilirubin in 32 patients, all of levels returned to the basal level soon. Duodenal ulcers were found in 11 patients, grade I in 8 patients and grade II in 3 patients, and soon return to basal levels after oral medication. Radiation-induced liver disease (RILD) developed in 4 patients (3.9%) over 3 months after completion of RT, and no death was found with symptomatic management.
Prognostic factors affecting overall survival
Statistical analysis results are shown in Table 2. Univariate analysis showed that ascites (P=0.001), different RT techniques (P=0.018), tumor size (P<0.001), TNM stage (P<0.001), Child-Pugh classification (P<0.001), TACE times (P=0.014), irradiation dose (P=0.016), mean dose of liver (Dmean) (P=0.002) and V20 (P=0.001) were the prognostic factors for overall survival (Figure 1). In this study, the tumor size was divided into two groups (d ≤6.1 cm and d >6.1 cm), the median survival time of group d ≤6.1 cm was 29 months, and of group d >6.1 cm was 18 months. Moreover, 1, 3, and 5 years survival rates in group d ≤6.1 cm to d >6.1 cm were 87.5% to 67.6%, 36.8% to 16.2%, and 10.9% to 2.7%, respectively (P<0.001). The median survival time of TNM stage II, III, and IV was 43, 25, and 15 months, moreover, 1-, 3-, and 5-year survival rates of patients with stage II were 100%, 48.3%, 20.7%, stage III were 80.4%, 21.7%, 4.3%; and IV were 57.7%, 11.5%, 0%. The TACE time was divided into two groups (n≤3 and n>3), the median survival time of group n≤3 was 22 months, and that of group n>3 was 29 months. The 1-, 3-, and 5-year survival rates in group n≤3 to group n>3 were 74.0% to 96.4%, 21.9% to 39.3%, and 2.7% to 21.4%, respectively (P=0.014). V20 was sub divided into two groups (V20 ≤45.41% and V20 >45.41%), the median survival time of group V20 ≤45.41% was 29 months, group V20 >45.41% was 18 months. The 1-, 3-, and 5-year survival rates of patients with group V20 ≤45.41% were 87.7%, 35.4%, 10.8%, respectively, and those of group V20 >45.41% were 66.7%, 11.1%, 2.8%, respectively.
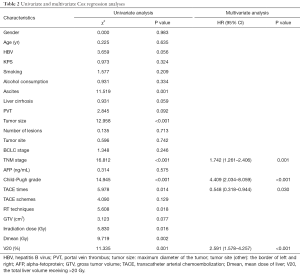
Full table
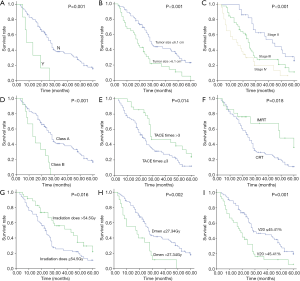
A Cox regression model was used to further assess the independent prognostic ability of the aforementioned risk factors. These factors were analysed by multivariate analysis. After adjustment for potential confounders, only the following prognostic factors were found to be independent: TNM stage [P<0.001, hazard ratio (HR), 1.742, 95% confidence interval (CI), 1.261–2.406], Child-Pugh grade (P<0.001, HR, 4.409, 95% CI, 2.034–8.059), TACE times (P=0.030, HR, 0.548, 95% CI, 0.318–0.944) and V20 (P<0.001, HR, 2.591, 95% CI, 1.578–4.257). Patients with early TNM stage, Child-Pugh class A, TACE times >3 and V20 ≤45.41% had a longer survival time.
Thereafter, for 26 HCC patients with PVT, univariate and multivariate analyses were performed to determine prognostic factors. We found that the PVT type was a relative factor for overall survival.
Discussion
With the increase in the incidence HCC, various methods have been used to treat HCC. It is critical to select the optimal treatment method and predict the prognosis of HCC. TACE is the preferred treatment for inoperable HCC, but patients are susceptible to relapse due to dual blood supply and branching vessels formed. It may cause liver damage due to hepatic ischemia when combined with PVT. A previous article showed that TACE was better suited as a bridge rather than a radical treatment (14). The combination of TACE and RT (3D-CRT and IMRT) can improve this situation. On one hand, RT can destroy residual tumor cells after TACE and the effect of subsequent TACE can be enhanced by inducing regression of the arteriovenous shunts around the PVT. On the other hand, the target volume after TACE was reduced and the tumor boundary was clear, that is conducive to the precise outline of the target, and the retention chemotherapy drugs in the liver also play a role in RT sensitisation.
Previous studies have confirmed that the efficacy of 3D-CRT combined with TACE was better than that of TACE alone in the treatment of uHCC (9,14,15), however, the independent factors affecting prognosis remain controversial. In this study, we analysed the different factors and drew some positive conclusions.
The irradiation dose and Dmean had no significant relationship with the overall survival rate in our study. This may be because of the heterogeneity of the tumor patient.
Although we did not find that PVT was a separate influencing factor, previous studies have suggested that it was an important prognostic factor (16-18). It is possible that RT maintains the portal vein blood flow by reducing intravascular tumor growth, preventing the deterioration of liver function, and limiting the spread of the tumor in the liver (19). In addition, patients with embolization in our study are mostly type I or II and the location of thrombosis influence prognosis (20). It was reported that patients with type I and II PVT yielded the best results with surgical treatment, whereas those type III PVT had the best effects with TACE + RT (21).
It is easy to understand that the TNM stage is a significant factor. In addition, Child-Pugh classification is an important predictor of the overall survival rate. In our study, 1-, 3-, and 5-year survival rates in Child-Pugh Class A to B of 80.2% to 66.7%, 30.3% to 0.0%, and 9.0% to 0.0%, respectively (P<0.001). The main reason for this large difference is that patients with poor liver function are less tolerant of radiation and prevent repair, which damaged by radiation and TACE. Moreover, Cheng reported that Child-Pugh class B would promote the incidence of RILD (22). In the present study, 4 patients developed RILD, and all of them were Child-Pugh class B. Therefore, it is necessary to consider liver function in the treatment of uHCC.
TACE has been used as a first-line treatment for advanced HCC for many years and could effectively prolong the survival of patients (4), but there are controversies about the number of TACE times. Most studies have shown that the number of TACE times was a protective factor for HCC (23). Because of arteriovenous fistula, the injected lipiodol will be lost over time. Repeated TACE can prolong the survival time by compensating for the incomplete effect of previous treatment (24). In our study, 1-, 3-, and 5-year survival rates of patients were significantly different among patients with ≤ 3 times (74.0%, 21.9%, and 2.7%, respectively) and patients with> 3 times (96.4%, 39.3%, and21.4%, respectively) (P=0.014). Because of the presence of arteriovenous fistula, the infusion of lipiodol may be lost, and repeated TACE can prolong the patient’s life expectancy by compensating for the incomplete effect of previous treatment (24). As long as liver function is maintained, TACE can be continued until the lipiodol is covered with all the tumor.
In addition, V20 significantly influenced the overall survival of patients independently. Liang et al. showed that the total liver volume receiving >10 Gy (V10) should be limited to <68%, V20 should be limited to <49%, and V30 should be limited to <28% in order to minimise the risk of RILD (25). In another study, Liang proved that V20 was an independent predictor of RILD for HCC patients (26). We did not analyse the correlation between V20 and RILD, because only 4 patients had RILD in our study, however, in my opinion, V20 was closely related to RILD. Our results were as follows: the overall survival rate at 1, 3 and 5 years of patients treated with V20 ≤45.41% were 87.7%, 35.4% and 10.8%, respectively, compared with 66.7%, 11.1%, and 2.8%, respectively in patients treated with V20 >45.41% (P=0.001). The median survival period of the two groups was 27 vs. 18.5 months. The present finding suggests that V20 >45.41% may reduce the survival of patients with uHCC. It is therefore necessary to consider V20 as a prognostic factor in clinical treatments and trials of uHCC patients.
Our study has some limitations. First, all patients we selected were treated with conventional RT, therefore, the results cannot be generalised to those treated with hypofractionated RT. Second, because biomarker measurement is not a routine check in our hospital except AFP, we did not analyse the other biomarkers on the influences of the overall survival. Third, the results shown pertain only to Child-Pugh class A and B patients. In addition, this research conclusion proves, to some extent, that the combination of TACE and RT is a promising treatment for uHCC. However, the strength of the evidence is weakened by the retrospective design, and more prospective studies must be conducted.
In summary, the TNM stage, Child-Pugh classification, TACE times and V20 are independent prognostic factors for overall survival. We hope that these findings will help provide patients with personalised treatment plans and yield a higher survival benefit. Future prospective studies are required to determine the efficacy and influencing factors of TACE plus RT in patients with uHCC.
Acknowledgments
Funding: This work was supported by National Key R&D Program of China (2017YFC0107502).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.04.22). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Ethics Committee of the Shandong Cancer Hospital and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- Bolondi L. Screening for hepatocellular carcinoma in cirrhosis. J Hepatol 2003;39:1076-84. [Crossref] [PubMed]
- Higuchi T, Kikuchi M, Okazaki M. Hepatocellular carcinoma after transcatheter hepatic arterial embolization. A histopathologic study of 84 resected cases. Cancer 1994;73:2259-67. [Crossref] [PubMed]
- Xu W, Kwon JH, Moon YH, et al. Influence of preoperative transcatheter arterial chemoembolization on gene expression in the HIF-1alpha pathway in patients with hepatocellular carcinoma. J Cancer Res Clin Oncol 2014;140:1507-15. [Crossref] [PubMed]
- Pan CC, Kavanagh BD, Dawson LA, et al. Radiation-associated liver injury. Int J Radiat Oncol Biol Phys 2010;76:S94-100. [Crossref] [PubMed]
- Kondo Y, Kimura O, Shimosegawa T. Radiation therapy has been shown to be adaptable for various stages of hepatocellular carcinoma. World J Gastroenterol 2015;21:94-101. [Crossref] [PubMed]
- Wu DH, Liu L, Chen LH. Therapeutic effects and prognostic factors in three-dimensional conformal radiotherapy combined with transcatheter arterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol 2004;10:2184-9. [Crossref] [PubMed]
- Zhou ZH, Liu LM, Chen WW, et al. Combined therapy of transcatheter arterial chemoembolisation and three-dimensional conformal radiotherapy for hepatocellular carcinoma. Br J Radiol 2007;80:194-201. [Crossref] [PubMed]
- Oh D, Lim DH, Park HC, et al. Early three-dimensional conformal radiotherapy for patients with unresectable hepatocellular carcinoma after incomplete transcatheter arterial chemoembolization: a prospective evaluation of efficacy and toxicity. Am J Clin Oncol 2010;33:370-5. [Crossref] [PubMed]
- Xu LT, Zhou ZH, Lin JH, et al. Clinical study of transarterial chemoembolization combined with 3-dimensional conformal radiotherapy for hepatocellular carcinoma. Eur J Surg Oncol 2011;37:245-51. [Crossref] [PubMed]
- Chen WJ, Yuan SF, Zhu LJ, et al. Three-dimensional conformal radiotherapy in combination with transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma. J BUON 2014;19:692-7. [PubMed]
- Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol 2001;35:421-30. [Crossref] [PubMed]
- Lencioni R, Llovet JM. Modified RECIST (mRECIST) Assessment for Hepatocellular Carcinoma. Semin Liver Dis 2010;30:52-60. [Crossref] [PubMed]
- Paik EK, Kim MS, Jang WI, et al. Benefits of stereotactic ablative radiotherapy combined with incomplete transcatheter arterial chemoembolization in hepatocellular carcinoma. Radiat Oncol 2016;11:22. [Crossref] [PubMed]
- Li B, Yu J, Wang L, et al. Study of local three-dimensional conformal radiotherapy combined with transcatheter arterial chemoembolization for patients with stage III hepatocellular carcinoma. Am J Clin Oncol 2003;26:e92-9. [Crossref] [PubMed]
- Llovet JM, Bustamante J, Castells A, et al. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology 1999;29:62-7. [Crossref] [PubMed]
- Lai EC, Lau WY. The continuing challenge of hepatic cancer in Asia. Surgeon 2005;3:210-5. [Crossref] [PubMed]
- European Association For The Study Of The Liver. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908-43. [Crossref] [PubMed]
- Yoon SM, Lim YS, Won HJ, et al. Radiotherapy plus transarterial chemoembolization for hepatocellular carcinoma invading the portal vein: long-term patient outcomes. Int J Radiat Oncol Biol Phys 2012;82:2004-11. [Crossref] [PubMed]
- Kim DY, Han KH. Transarterial chemoembolization versus transarterial radioembolization in hepatocellular carcinoma: optimization of selecting treatment modality. Hepatol Int 2016;10:883-92. [Crossref] [PubMed]
- Wang K, Guo WX, Chen MS, et al. Multimodality Treatment for Hepatocellular Carcinoma With Portal Vein Tumor Thrombus: A Large-Scale, Multicenter, Propensity Mathching Score Analysis. Medicine 2016;95:e3015 [Crossref] [PubMed]
- Cheng JC, Wu JK, Lee PC, et al. Biologic susceptibility of hepatocellular carcinoma patients treated with radiotherapy to radiation-induced liver disease. Int J Radiat Oncol Biol Phys 2004;60:1502-9. [Crossref] [PubMed]
- Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74-108. [Crossref] [PubMed]
- Ikeda K, Kumada H, Saitoh S, et al. Effect of repeated transcatheter arterial embolization on the survival time in patients with hepatocellular carcinoma. An analysis by the Cox proportional hazard model. Cancer 1991;68:2150-4. [Crossref] [PubMed]
- Liang SX, Zhu XD, Xu ZY, et al. Radiation-induced liver disease in three-dimensional conformal radiation therapy for primary liver carcinoma: the risk factors and hepatic radiation tolerance. Int J Radiat Oncol Biol Phys 2006;65:426-34. [Crossref] [PubMed]
- Liang SX, Huang XB, Zhu XD, et al. Dosimetric predictor identification for radiation-induced liver disease after hypofractionated conformal radiotherapy for primary liver carcinoma patients with Child-Pugh Grade A cirrhosis. Radiother Oncol 2011;98:265-9. [Crossref] [PubMed]


