A rare unilateral giant fibroadenoma of the breast during pregnancy and lactation
Introduction
Giant fibroadenoma (GFA) accounts for 2% to 10% of all fibroadenomas in breasts. GFA is referred to when a tumor reaches a size larger than 5 cm in diameter and/or weighs more than 500 g. GFA is an uncommon variant of fibroadenoma and the most common cause of unilateral macromastia in adolescents (1). Pregnancy is associated with profound changes in maternal breasts. A hormonal milieu of pregnancy induces lobular and alveolar proliferative changes. During pregnancy, in response to hormonal stimulus, fibroadenomas often increase in size and occasionally become infarcted, causing a significant increase in size and pain, which may often be confused with cancer. Physiologic changes associated with pregnancy make it difficult to detect small lesions (2). However, when GFA causes significant deformity and suspicion of malignancy, surgical excision is required (3). We presented a case of GFA in the left breast during pregnancy and lactation.
Case presentation
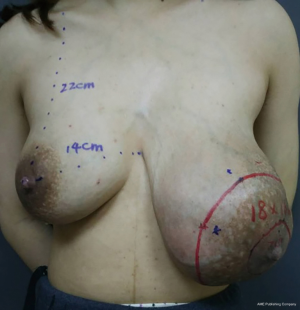
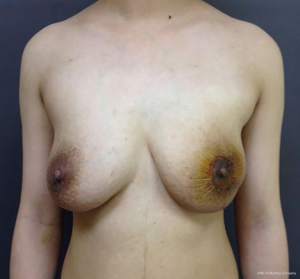
A 27-year-old woman in the second week of lactation was referred to our clinic because of an enormous lump in her left breast. She was admitted to the hospital on April 25, 2016. The patient stated that she palpated the lump about 6 months ago; at that time, she was 4 months pregnant, and the lump grew more than twofold during the course of the pregnancy. Her family history was negative for breast cancer. Physical examination revealed a solid and mobile left tumor with a size of more than 15 cm. The tumor distorts the contour of the left breast, thereby causing significant breast asymmetry (Figure 1). No suspicious lymph nodes were detected.
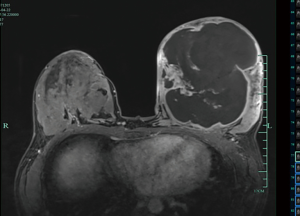
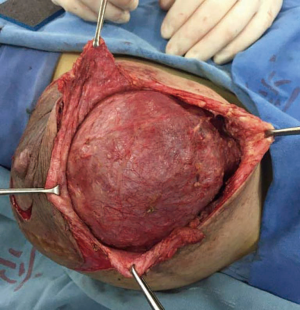
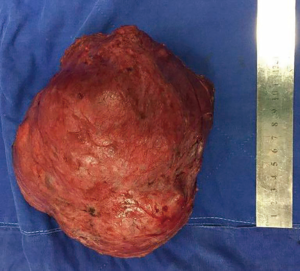
Breast ultrasonography demonstrated a hypoechoic solid mass with partial liquid content, and magnetic resonance imaging showed an inhomogeneous tumor almost occupying the entire left breast (Figure 2). A core biopsy was performed showing adenosis of the mammary gland and usual ductal hyperplasia. These findings were discussed with the patient in detail, and she signed all informed consent forms. A lumpectomy was indicated because of the critical deformity of the left breast. Vitamin B6 plus bromocriptine tablet was prescribed to suppress lactation pre- and post-operation. The operation was performed under general anesthesia. A round block incision was chosen as surgical access because of possible removal of redundant skin and to obtain a discrete postoperative scar. The area between the areola and the peripheral outline was carefully de-epidermized to keep the underlying dermis intact. The tumor was easily enucleated to preserve the nipple and areola complex (Figure 3). Moreover, postoperative pathology results revealed a fibroadenoma with massive necrosis (Figure 4). The tumor size was determined to be 17.5×15.5×9.5 cm3 with a final weight of 1,482 g. No prominent deformity was observed after the operation.
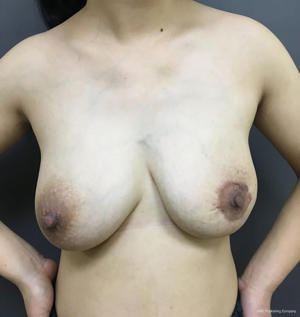
The patient was discharged from hospital on the fifth postoperative day with fine wound healing and returned to the clinic after one month with a good cosmetic outline (Figure 5).
Two years after the operation, the patient returned for re-examination by which she was pregnant at 4 months. No recurrence was found by breast ultrasonography, and symmetrical breast shape was maintained (Figure 6).
Discussion
In addition to milk stasis, mastitis, and lactational breast abscesses, pregnant women may develop other breast problems seen in nonpregnant women (2). Fibroadenomas often increase in size during pregnancy and lactation; higher concentrations of estrogen, progesterone, and prolactin might be the causes for their significant enlargement (4). As a result of rapid growth, infarction can occur during pregnancy and lactation, which is generally asymptomatic (2).
Most common mass lesions during lactation are benign. Mammography may be essential; however, increased density and water content of breasts make it difficult to detect small lesions. Ultrasonography is useful in differentiating cystic from solid lesions. For highly suspicious masses, a core needle or excisional breast biopsy remains the best approach (5). A fine-needle aspiration cytology yielding a false-negative rate of up to 10–20% is insufficient. A large-core needle biopsy to obtain adequate tissue for diagnosis may have a lower incidence of false-negative results, possibly even approaching zero. However, other reports suggest that core-needle biopsy is generally not obligatory for tissue diagnosis because histological results rarely influence the recommendation for surgical excision. For differential diagnosis between GFA, phyllodes tumor, and hematoma or malignancy, core-needle biopsy may be helpful (5,6). An excisional biopsy should be the last resort in establishing a confirmed diagnosis (3,7). Lactation should be suppressed before biopsy to prevent milk fistula. Increased incidence of milk fistula and breast abscess might occur if surgery is performed while breast feeding continues (2).
Moreover, masses consistent with fibroadenoma on physical examination and imaging could be managed nonsurgically. Given their size, progressive growth, associated pain, and esthetic concerns, GFAs may be more clinically alarming. Rapid growth, distortion of breast architecture or with overlying skin changes, and risk of malignancy are indications for surgical excision of a suspected fibroadenoma (5,6). An ideal surgical technique for GFA with asymmetry should achieve equal breast size, reposition the nipple-areola complex, and correct asymmetry with minimal scars in one stage. Multiple treatment modalities ranging from simple excision to mastectomy have been described in the literature (3,8). Mastectomy may be considered a drastic option in benign breast lesions. The most common incisions reported for excision of GFAs are inframammary skin incision, reduction mammaplasty incision, and peri-/circum-areolar incision (3,9-12).
This case report illustrated a young female patient who presented with a distorted left breast due to a giant fibroadenoma during pregnancy and lactation. We treated our patient using the round block technique described by Benelli (13). This technique permits easy access to a tumor while leaving a very discrete scar and allows restoration of a good breast contour after excision.
Acknowledgments
Funding: This work was supported by Hangzhou Science and Technology Bureau [20170533B26].
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.05.10). The authors have no conflicts of interest to declare.
Ethical statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chang DS, McGrath MH. Management of benign tumors of the adolescent breast. Plast Reconstr Surg 2007;120:13e-9e. [Crossref] [PubMed]
- Scott-Conner CE, Schorr SJ. The diagnosis and management of breast problems during pregnancy and lactation. Am J Surg 1995;170:401-5. [Crossref] [PubMed]
- Ugburo AO, Olajide TO, Fadeyibi IO, et al. Differential diagnosis and management of giant fibroadenoma: comparing excision with reduction mammoplasty incision and excision with inframammary incision. J Plast Surg Hand Surg 2012;46:354-8. [Crossref] [PubMed]
- Heilmann T, Leuschner I, Hilpert F, et al. Diagnosis and management of an unilateral giant fibroadenoma of the breast in pregnancy. Arch Gynecol Obstet 2012;285:235-7. [Crossref] [PubMed]
- Cerrato FE, Pruthi S, Boughey JC, et al. Intermediate and long-term outcomes of giant fibroadenoma excision in adolescent and young adult patients. Breast J 2015;21:254-9. [Crossref] [PubMed]
- Jayasinghe Y, Simmons PS. Fibroadenomas in adolescence. Curr Opin Obstet Gynecol 2009;21:402-6. [Crossref] [PubMed]
- Gordon PB, Gagnon FA, Lanzkowsky L. Solid breast masses diagnosed as fibroadenoma at fine-needle aspiration biopsy: acceptable rates of growth at long-term follow-up. Radiology 2003;229:233-8. [Crossref] [PubMed]
- Sosin M, Feldman E. Giant juvenile fibroadenoma: a case and review of novel modalities in treatment. Breast Dis 2012;34:35-8. [Crossref] [PubMed]
- Achebe JU, Njeze GE, Okwesili OR. Treatment of unilateral giant fibroadenoma by breast reduction skin incision: the inverted "T" technique. Niger J Clin Pract 2014;17:43-6. [Crossref] [PubMed]
- Park CA, David LR, Argenta LC. Breast asymmetry: presentation of a giant fibroadenoma. Breast J 2006;12:451-61. [Crossref] [PubMed]
- Ciftci I, Sekmenli T, Ozbek S, et al. Inframammarial Giant Fibroadenoma Removing and a Nipple-sparing Breast Reconstruction in an Adolescent: A Case Report. Prague Med Rep 2015;116:161-6. [Crossref] [PubMed]
- Camara O, Egbe A, Koch I, et al. Surgical management of multiple bilateral fibroadenoma of the breast: the Ribeiro technique modified by Rezai. Anticancer Res 2009;29:2823-6. [PubMed]
- Benelli L. A new periareolar mammaplasty: the "round block" technique. Aesthetic Plast Surg 1990;14:93-100. [Crossref] [PubMed]