
Neoadjuvant therapies for surgical management of high-risk, localized prostate cancer
Introduction
Along with external beam radiation therapy (XRT) with concomitant androgen deprivation therapy (ADT), radical prostatectomy (RP) is a first-line treatment for high-risk, clinically localized prostate cancer. These patients have negative staging exams (e.g., nuclear bone scan, cross sectional imaging) and presumed local or loco-regional disease. Goals of surgical treatment are cure through eradication of macro- and microscopic cancer, pathological staging to determine the need for adjuvant therapies such as pelvic XRT +/− ADT, and local control to reduce risk of urinary tract obstruction, gross hematuria, etc. should the patient develop advanced disease. High-risk disease is conventionally defined using D’Amico criteria, which include prostate-specific antigen (PSA) ≥20 ng/dL, Gleason score 4+4=8 (Grade group 4) or higher, and clinical stage ≥T2c. These patients often have aggressive disease phenotypes, reflected in greater risk of metastatic progression and death without treatment, greater risk of recurrence following local treatment, and greater need for adjuvant or salvage therapies (1,2). While traditionally XRT + ADT has been used for high risk patients based on concern for the ineffectiveness of surgery, RP has been increasingly used for localized, high risk disease (3,4) with principles of wide resection and extended lymphadenectomy to achieve the objectives detailed above. Retrospective series have demonstrated the effectiveness of surgery similar to XRT (5).
Given higher risks of relapse among patients treated for high-risk disease, there has been ongoing effort to develop strategies to improve outcomes for these patients, including following surgical management. Adjuvant or early salvage therapies are commonly employed for high-risk RP patients based on concerning findings on surgical pathology (e.g., extraprostatic disease, lymph node involvement) or biochemical recurrence, respectively. These therapies have been shown to reduce risk of clinical progression and potentially improve survival, e.g., adjuvant XRT for extra-prostatic disease, adjuvant ADT in lymph node positive patients (6,7). To prospectively improve the effectiveness of surgical treatment, there has been ongoing research in neoadjuvant therapies for high-risk patients undergoing RP. Goals of neoadjuvant therapy are several-fold and include downstaging cancer and improving resectability, improving pathological outcomes which may correlate with downstream survival, treating microscopic distant disease that would otherwise blossom post-local treatment, and ultimately to improve survival related to prostate cancer. There is widespread precedent for neoadjuvant systemic therapy in management of other malignancies, such as breast, bladder, rectal and other cancers, fueling enthusiasm for this approach in the treatment of high-risk prostate cancer (8,9).
In this article, we present a summary of contemporary evidence for neoadjuvant systemic therapies in the management of clinically localized high-risk prostate cancer, including hormonal, chemotherapeutic, and novel agents. While none of these regimens are currently standard of care, data are emerging from varied trials including RCTs regarding the efficacy of these therapies.
Neoadjuvant ADT
Given the hormone sensitivity of most newly diagnosed prostate cancers, neoadjuvant ADT has been studied to improve surgical outcomes for high-risk patients. Unfortunately, neoadjuvant ADT as monotherapy has failed to show a compelling clinical or survival benefit in multiple studies. It has been suggested that lack of effect of neoadjuvant ADT may relate to the presence of castrate-resistant cells at the time of diagnosis, or developing during treatment (10). Notably, neoadjuvant ADT with XRT (concomitant, but starting prior to XRT) for intermediate-high-risk cancer has demonstrated improved biochemical recurrence-free survival (BCRFS), likely related to the different biological effects of radiation vs. surgery (11).
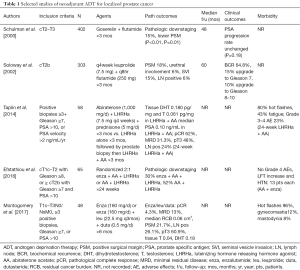
One of the largest preliminary trials investigating neoadjuvant hormonal therapy (NHT) prior to RP was conducted by Schulman et al. in 2000 (12). Their study included 402 patients with cT2-T3 prostate cancer who were randomized to preoperative goserelin plus flutamide for 3 months prior to RP versus RP alone. The follow-up period was 4 years. They found higher rates of pathologic downstaging in the NHT group compared to the RP alone group (15% vs. 7%, P<0.01). In both cT2 and cT3 groups, the rates of positive surgical margin were lower in those who received NHT (cT2 P<0.01, cT3 P=0.01). However, there was a similar PSA progression rate between groups (P=0.18).
Another large study by the Lupron Depot Neoadjuvant Prostate Cancer Study Group was a prospective, multi-institutional trial including 303 patients with stage cT2b prostate cancer who were randomized to RP only or 3 months of neoadjuvant ADT (monthly leuprolide + q8hr flutamide) followed by RP. Patients in the NHT group had a greater decrease in prostate volume and preoperative PSA, lower rates of positive surgical margin (18% vs. 48%, P<0.001) and lower rates of urethral margin involvement (6% vs. 23%, P<0.01), however at 5 years, there was no difference in the biochemical recurrence rate (64.8% vs. 67.6%, P=0.663) (13).
While there are many similar studies, in 2009 Shelley et al. published a systematic review and meta-analysis of ten randomized trials of NHT prior to RP for localized and locally advanced prostate cancer. They found that NHT was associated with lower pathological T stage, higher rates of organ confinement, lower rates of positive surgical margins and lower rates of lymph node positivity. Positive surgical margin rates and organ confined rates were significantly improved with longer duration of neoadjuvant therapy. However, there was not an associated decrease in biochemical recurrence-free survival, progression-free survival, or overall survival (14).
There have been investigations of more novel androgen deprivation therapies in the neoadjuvant setting that have shown benefit histopathologically, but without study design to show a significant clinical effect. Taplin et al. randomized 58 patients with newly diagnosed localized intermediate- or high-risk prostate cancer (positive biopsies ≥3, Gleason ≥7, PSA >10, or PSA velocity >2 ng/mL/year) to 12 weeks of preoperative LHRHa alone (leuprolide acetate) or LHRHa plus abiraterone acetate (AA). All patients subsequently received an additional 12 weeks of LHRHa + AA before RP for 24 weeks of treatment. They found that levels of intraprostatic androgens from 12-week prostate biopsies were significantly lower in the LHRHa + AA group compared to the LHRHa alone group. When examining prostatectomy specimens at 24 weeks, the rates of pathological complete response (pCR) and minimal residual disease (MRD) were greater in the LHRHa + 24-week AA group compared to the LHRHa + 12-week AA group (90% vs. 48%). Many patients in both groups had T3-residual tumor (48% vs. 59% in LHRHa + 24-week AA vs. LHRHa + 12-week AA) and lymph node positivity (24% vs. 11% in LHRHa + 24-week AA vs. LHRHa + 12-week AA). The adverse events in both groups were comparable and there were no increased complications from prostatectomy after neoadjuvant therapy in either group (15).
Efstathiou and colleagues also published their preliminary results studying neoadjuvant enzalutamine (enza) + AA + LHRHa versus AA + LHRHa in patients with localized high-risk prostate cancer (cT1c-T2 with Gleason ≥8, or ≥ cT2b with Gleason ≥7 and PSA >10). Sixty-five patients were randomized 2:1 to receive 24 weeks of neoadjuvant enza + AA + LHRHa or AA + LHRHa prior to RP. Pathologic downstaging occurred in 30% of patients in enza + AA + LHRHa vs. 52% of patients in the AA + LHRHa group (P=0.07), suggesting no benefit to adding enza to augment the efficacy of AA + LHRHa in localized high-risk prostate cancer (16).
Finally, Montgomery et al. examined 48 patients with intermediate- or high-risk localized prostate cancer (surgically resectable, T1c-T3N0/NxM0, ≥3 positive biopsies, Gleason ≥7, or PSA >10) who received neoadjuvant enzalutamide (enza) or enzalutamide/dutasteride/leuprolide (enza/dut/LHRHa) for 6 months prior to prostatectomy. They found that none of the patients in the enza group achieved pCR or MRD. In the enza/dut/LHRHa group, 4.3% achieved pCR and 13.0% achieved MRD. The median residual cancer burden (RCB) was higher in the enza group and tissue testosterone and DHT levels correlated with RCB. In both groups, extensive nuclear androgen receptor (AR) staining remained in the majority of tumor cells in all residual tumors, suggesting incomplete suppression of AR expression. There were no adverse events leading to drug discontinuation in either group (17).
These studies represent early work in the evaluation of novel, and more aggressive androgen blockade in the neoadjuvant setting. Further work is needed in more rigorous formats, with clinical endpoints and longer term follow-up, to evaluate which agents or combination of agents may have benefit.
Neoadjuvant chemotherapy and chemohormonal therapy
As neoadjuvant ADT has failed to improve surgical outcomes for high risk patients, chemotherapy and combined chemohormonal therapy have been investigated in this space. An agent that has been heavily studied in the neoadjuvant setting, though primarily in phase I and II studies, is docetaxel (D), both alone and in combination with ADT. Docetaxel is a taxane antineoplastic that works by targeting the nuclear matrix and microtubular function. This agent has been pursued based on its activity and benefit in advanced prostate cancer. Indeed, D + ADT has been an important regimen within the treatment algorithm for castrate-resistant prostate cancer (18,19), and more recently, D+ADT has proven efficacious in initial management of castrate-sensitive disease (CHAARTED). Docetaxel is typically combined with ADT to optimize treatment of both androgen-dependent and -independent cell lines within a tumor; animal models have shown that D effectively treats androgen-independent clones at an early point in disease progression (20). Interestingly, in vitro studies have shown that even in androgen dependent tumor models, simultaneous chemotherapy + ADT can be more effective than sequential treatment (21).
Evidence for the efficacy of docetaxel-based neoadjuvant therapy is early and evolving. Trials have primarily examined safety and feasibility of chemotherapy and chemohormonal therapy regimens. Common endpoints in these studies have been pathological outcomes, such as pCR rates, as this is considered a proxy outcome for oncological efficacy. pCR is associated with favorable long term outcomes in treatment of some solid malignancies, e.g., breast (8) and bladder (22), though survival benefit does not require this pathological feature to occur. It is not clear if more detailed pathological analysis such as quantification of necrosis, apoptosis, proliferation, etc. would be useful endpoints (23). While phase II studies have limited effect on clinical practice, there are phase III studies that are ongoing or concluded and not yet reported. Limitations of the existing evidence, some inherent to their design and others methodological, include short follow-up, low numbers of enrolled patients, heterogeneous patient populations (e.g., variability in inclusion criteria and definitions of ‘high risk’, such that some intermediate patients may be included), varied regimens (e.g., weekly vs. q3 week docetaxel, shorter vs. longer treatment courses), and lack of rigorous clinical endpoints. It is important to contextualize conclusions from these studies, as we have learned from neoadjuvant ADT studies that favorable pathological outcomes (e.g., lower rates of positive surgical margins) have not translated to improved oncological outcomes.
Neoadjuvant chemotherapy
As stated above, D has been scrutinized as a neoadjuvant therapy for high risk RP based on its activity in the advanced cancer setting. There have been some favorable proxy outcomes of neoadjuvant D including PSA declines pre-surgically, however there have been no reported pCR outcomes with D alone. Mostly, in the investigative setting, D has been jettisoned in favor of a combined D + ADT approach as discussed below. Insight into lack of robust responses to D monotherapy may come from molecular and histological studies of cytotoxic effects of D, in which there have been complex and discordant findings (e.g., upregulation of p53, Bcl-2, and increased expression of Ki67 in the same patients) that may be attributed to clonal heterogeneity in prostate cancer cells and complex pathways for apoptosis (24).
Some examples of studies evaluating effects of neoadjuvant D, and D + other chemotherapies, follow. Febbo et al. studied 19 patients with high risk cancer (Gleason 8–10, PSA >20, and/or cT3) who received weekly docetaxel (36 mg/m2) ×6 months followed by RP (25). PSA decline >50% occurred in 58%, MRI showed maximum tumor volume reduction of at least 25% in 68%, and at least 50% in 21%. Sixteen patients went on to RP, and none had pCR. These authors evaluated gene expression within local tumors post-treatment, and found a consistent increase in expression of androgen metabolism genes leading to a decrease in active androgens available to prostate cancer cells. These authors postulated that prostate cancer cells that survive docetaxel have altered androgen metabolism, leading to lower sensitivity to hormone deprivation, another potential mechanism for lack of efficacy. RNA expression of enzymes that decrease cellular levels of bioactive androgens were also increased in response to chemotherapy. This is a potential concern in docetaxel causing resistance to ADT through changes in androgen metabolism, but this requires additional study.
Zhao et al. reported long term survival of patients with locally advanced CaP (cT2b+, biopsy Gleason 8+, and/or PSA 15+) who received 6 weekly doses of docetaxel (40 mg/m2) + RP. These authors found that at a median follow-up of 130 months [37–166], 18 patients (64%) had BCR while 10 patients (36%) were alive and free of disease. CSS was 92.2%, and overall survival 79.7%, though this study was not powered for survival (10).
Garzotto et al. (26) published a phase I/II study of neoadjuvant docetaxel and mitoxantrone for high risk patients (cT2c-3a, PSA 15+, Gleason 4+3+), the latter being a cytotoxic chemotherapy with a different mechanism (suppressing proliferation of T cells, B cells, and macrophages, impairing antigen presentation, and decreasing secretion of pro-inflammatory cytokines), with the potential of synergistic activity against prostate cancer. Fifty-four patients received 4 cycles of both medications prior to RP. This study primarily showed feasibility and safety of this regimen, with some high-grade neutropenia (18/57) and leukopenia (21/57), though reasonable short-term outcomes. Through median 63 months follow up, 47.4% (27) experienced a recurrence, relapse-free survival at 5 years was ~50%. These authors looked molecularly at prostate tissue to evaluate predictors of recurrence or relapse. They found that increased tissue VEGF was an independent predictor of relapse, which supported the theoretical use of alternative agents like bevacizumab, a VEGF antibody (26). In a recent update of this study, 37% of patients were free of disease at 10 years, and VEGF expression, lymph node status, and PSA density were predictors of disease recurrence. The authors commented that there were no association between immunophenotype and recurrence, though was a significantly different density of CD68 and CD163 cells between normal and tumor tissue, the significance of which was not clear (28).
Clark et al. (29) reported a phase II study of neoadjuvant estramustine + etoposide + RP for locally advanced prostate cancer. Patient characteristics including clinical stage T2b-3, PSA ≥15 ng/dL, and Gleason ≥8. The treatment regimen including 3 cycles of estramustine (10 mg/kg/day) and etoposide (50 mg/m2/day) orally on days 1–21, every 28 days ×3. Eighteen patients completed chemotherapy, and 16 underwent RP. Pathological outcomes including 0% pCR, and 69% (13) had non-organ confined disease. Through short-term follow-up (median 14 months; range, 5–20 months) without additional therapy, 14 patients who had negative pathological lymph nodes were free of disease. Those with positive lymph nodes received ADT. The regimen had increased morbidity, primarily due to estramustine; there were 2 deep venous thrombosis (DVT) and one pulmonary embolism (PE).
Finally, Konety and colleagues evaluated 36 patients with clinically localized high risk disease (≥ cT3, Gleason ≥8, PSA ≥20 ng/dL) undergoing neoadjuvant paclitaxel, carboplatin, and estramustine (4 cycles) followed by RP. DVT occurred in 22% related to estramustine, however at median follow-up 29 months [5–51], 45% were free of BCR. The positive surgical margin rate was low at 22%. Long term outcomes were not assessed, e.g., clinical recurrence and survival (27).
Neoadjuvant chemohormonal therapy
Neoadjuvant combined D + ADT has been studied with the goal of treating both androgen-dependent and independent cells, both locally and systemically, prior to RP. These regimens have shown safety, feasibility, and activity at the pathological level. We await phase III trial data regarding efficacy at this time.
There are multiple phase II studies that have yielded encouraging early outcomes regarding neoadjuvant D + ADT. One example is Chi et al. (30) reported a phase II multicenter trial of 72 high risk localized patients receiving neoadjuvant ADT + docetaxel (35 mg/m2 weekly for 6–8 weeks ×3). Clinically, 39% had extraprostatic disease (cT3), and 60% were high grade (Gleason ≥8). Sixty-four patients went on to RP (4 withdrew due to toxicity), and 2 had pCR. There were some favorable pathological outcomes, including 16 patients with <5% tumor volume, 3 with microfoci of disease only, suggesting downstaging in a subset of patients. Pathologically, 44% had pT3 disease, 4 were pN1, and there was a PSM rate of 27%. Oncological outcomes included 30% disease relapse at median 42.7 months of follow-up.
The results from a large RCT of D + ADT vs. surgery alone are pending. This study, called CALGB 90203 (Cancer and Leukemia Group B), a phase III study comparing neoadjuvant docetaxel + ADT with surgery alone for high risk localized cancer. The primary outcome is 3-year biochemical progression-free survival (bPFS), with secondary outcomes of 5-year bPFS, clinical recurrence, metastatic progression, cancer-specific and overall survival. 750 patients with high risk disease have randomized to RP vs. 6 cycles of docetaxel (75 mg/m2 every 3 weeks) and 18–24 weeks of ADT + RP. Inclusion criteria include a Kattan nomogram probability of freedom from biochemical progression at 5 years of <60%, or biopsy Gleason sum 8 or greater, with negative staging exams.
An early pathological report from this trial was recently reported, and provides some interesting insight into mechanisms of activity and resistance. At one center within the trial, there were 3 of 52 patients with only micro-focal residual cancer. Gene alterations in treated cells varied (TMPRSS2-ERG fusion in 32, TP53 mutation or deletion in 11, PTEN deletion in 5, FOXA1 in 6, and SPOP mutation in 4). There was no androgen receptor (AR) amplification or mutations, there was up-regulation of AR and AR-V7 expression as well as other genes, and the degree of AR signaling suppression varied among specimens. This study shows the varied effects of treatment at a molecular level that may ultimately give insight into early markers of resistant disease (31).
Outside the scope of this paper but worth cursory discussion, there has been some investigation of neoadjuvant D + ADT in patients with clinically positive lymph nodes, with the hope that consolidative therapy may improve outcomes, akin to the paradigm for bladder cancer. Zurita et al. (32) reported that among 36 patients with cN+ disease or high risk of nodal spread receiving neoadjuvant docetaxel + ADT, 4 had progression during treatment and 4 did not nadir PSA <1. Twenty-six (67%) underwent RP, and 13 (33%) were free of progression at 1 year. Mean follow-up was 61 months, at which time ½ of patients were free of progression and off treatment at 1 year after surgery, suggesting that some patients may have benefited from therapy. Outcomes were still adverse with >75% with residual locally advanced disease post-neoadjuvant therapy, and ~50% with pathological nodal metastasis. There was no control arm of men with alternative treatment approaches (e.g., ADT for pathologically positive LN), and this study only showed feasibility, however this is another space in which further investigation is needed. Furthermore, this study underscores an important point that clinical response to neoadjuvant therapy may select patients most likely to benefit from surgery, though criteria for “appropriate response” are poorly defined.
There have been studies of other chemohormonal regimens that merit review. These have primarily been early phase II studies that have not stimulated randomized trials. Docetaxel + estramustine has been studied as a neoadjuvant regimen as this is an active combination in castrate-resistant prostate cancer (33). Thromboembolism risk from estramustine, however, has limited enthusiasm for this agent. One phase II trial (GETUG-12) randomized 413 patients to ADT ×3 years (goserelin 10.8 mg q3 months) plus 4 cycles docetaxel (70 mg/m2) + estramustine (10 mg/kg daily on days 1–5, q3 weeks), vs. ADT alone, followed by local therapy, primarily XRT (87%) but also RP (13%). Patients had clinical stage T3–T4 disease, Gleason score of ≥8, PSA >20 ng/mL, or clinical node-positivity. All patients underwent a staging pelvic lymph node dissection prior to therapy. Relapse-free survival was superior at 8 years for combination therapy vs. ADT only (62% vs. 51%; P=0.017); HR 0.71 (0.54–0.94), though there was insufficient data for determination of metastasis-free and overall survival (34).
There are small series of ADT + docetaxel + estramustine that have shown safety and feasibility, with some patients experiencing a complete pathological response (35,36). There has been long term follow-up in one phase II trial assessing outcomes of neoadjuvant ADT + paclitaxel, carboplatin, and estramustine, with comparison to a contemporary cohort meeting inclusion criteria but receiving surgery only (37). This study showed that among 34 patients, with a median follow up of 13.1 years, most patients experienced biochemical recurrence (78% within 10 years), however there was a high rate of DSS and OS at 10 years (84%, 78%, respectively). The study cohort had higher risk features, and with adjustment there was no significant difference in recurrence or metastasis between groups. Neoadjuvant estramustine + ADT has been examined retrospectively vs. RP alone for high risk patients. Koie et al. studied 274 patients with neoadjuvant therapy vs. matched pairs undergoing RP only with propensity score matching to address confounding. Five-year BCRFS was improved with neoadjuvant therapy (65.8% vs. 90.4%; P<0.0001) with no difference in OS (96% vs. 100%; P=0.110) (38).
There is phase II evidence of safety and tolerability of docetaxel + ketoconazole. Ketoconazole blocks production of testicular and adrenal androgens by inhibiting enzymes in the steroid synthesis pathway. This agent has been considering promising due to antiproliferative effects, synergy with chemotherapies like docetaxel, and activity versus CRPC (39). Furthermore, as docetaxel is metabolized by the cytochrome p-450 system, it is thought that ketoconazole may potentiate its activity. Womble et al. (40) enrolled 22 patients with localized high risk disease, who underwent neoadjuvant docetaxel and ketoconazole prior to RP. Nineteen patients went on to surgery, of which 36% were biochemically free of disease at median 18 months f/u. Six patients had salvage therapy, with an undetectable PSA. While there was grade 3–4 toxicity in most patients, primarily neutropenia (45%), 73% of patients completed all four courses of chemotherapy. There were no patients with pCR. 7 patients underwent adjuvant therapy, of whom 6 ad undetectable PSA at 18 months median f/u. As most patients had BC relapse and 47% had EPE and 26% had LN+, not promising enough to warrant RCTs (40).
Clinical effects of neoadjuvant therapy
Most studies of neoadjuvant chemo- or chemohormonal therapy discuss side effects, generally with focused discussion of higher grade hematological toxicities. See Tables 1,2 for example rates with different regimens. However, there is limited discussion of changes in operative findings or operative risk following neoadjuvant therapies. One study reported periprostatic fibrosis (23% of patients in one study) and increased diffuse bleeding (41), however in general there have not been additional surgical complications that have been reported. A recent analysis of patients undergoing neoadjuvant therapies showed a rate of significant and overall perioperative complications similar to those not receiving neoadjuvant therapy (42). Importantly, this is a population at risk for worse functional outcomes based on advanced disease and the requirement for wide resection of neurovascular bundles in many cases. Generally, however, there are reports of recovery of continence and erectile function similar to what would be expected for treatment of other high-risk patients (41).
Full table

Full table
Neoadjuvant chemotherapy + immunotherapy
Immunotherapy (e.g., bevacizumab) plus chemotherapy has been shown to improve clinical outcomes in other solid tumors (43), and to have activity against metastatic CRPC (44). Thus, this regimen has been studied in the neoadjuvant setting for high risk prostate cancer patients. Ross et al evaluated the efficacy of a single arm neoadjuvant docetaxel plus bevacizumab regimen pre-RP for high risk, localized patients (45). Bevacizumab is a humanized monoclonal antibody that binds to and neutralizes serum vascular endothelial growth factor (VEGF), which mediates tumor angiogenesis. Of 41 patients, clinical activity was demonstrated with a >50% reduction in tumor volume on MRI in 29%, and >50% reduction in PSA in 22%. Most patients underwent RP, and there were no complete responses. The regimen was safe, with 7% (3/41) having febrile neutropenia, and 93% of patients completing all cycles. There is some thought that antiangiogenic therapy may be more useful in bulky metastatic disease, by achieving vascular normalization and improved chemotherapy delivery, versus micrometastatic disease (44). Further study of subsets of patients and molecular stratification of response to treatment may enable more targeted use or study of these agents.
Other immunotherapies such as tyrosine kinase inhibitors (TKIs) have been studied in the neoadjuvant setting. These have had modest results and have not been considered promising agents (46). Vuky reported 31 high risk patients (cT2b+, PSA >20 ng/dL, Gleason ≥8) given neoadjuvant docetaxel + gefitinib (a small-molecule TKI of EGFR) for 2 months prior to RP. Of 30 patients undergoing RP (one had LN+ and T4 disease, and was excluded), there was 0% pCR rate, however 94% had a partial clinical response. At mean follow-up of 28 months, 67% (22) had no biochemical recurrence (47). Another TKI that has been studied is imatinib (versus PDGFR) plus docetaxel and ADT; there were no pCRs, and 53% of patients were free of biochemical disease at median 39 month follow-up, however in a patient group that included some at intermediate risk by D’Amico criteria (48).
Novel neoadjuvant therapies
A recent study explored the feasibility of in situ gene therapy as neoadjuvant therapy for high risk disease. Kumon et al. reported a phase I/IIa study of in situ gene therapy using an adenovirus vector carrying the human REIC/Dkk-3 gene (Ad-REIC). This gene is significantly reduced in various cancers, and forcing its expression may induce cancer-selective apoptosis. Among 18 high-risk patients, an ultrasound was used with MRI guidance to inject tumor six weeks prior to RP. Clear cytopathic effects were seen in a subset of patients (apoptosis, cellular degeneration), with infiltrations of CD8+ lymphocytes and dendritic cells, as well as a detected systemic effect. This regimen was well tolerated without significant complications. Interestingly, these authors had published a report of a patient with CRPC who had a direct and indirect systemic effects of Ad-REIC injection into metastatic lymph nodes (49,50).
There are numerous trials evaluating the role of novel therapeutics as neoadjuvant therapy. See Table 3 for ongoing trials using novel therapeutics including targeted agents and immunotherapies.

Full table
Conclusions
RP for high risk, clinically localized prostate cancer can be effective, but high recurrence rates have spurred investigations into neoadjuvant therapies. While neoadjuvant ADT has been shown to improve pathological outcomes from RP, lack of oncological benefit has precluded its use as standard of care. Study of novel hormonal agents in the neoadjuvant setting, such as abiraterone and enzalutamide, is early and additional work is needed to understand the potential role of these agents. There is encouraging evidence for the clinical benefit of neoadjuvant chemohormonal therapy (i.e., D + ADT), though level 1 evidence is pending. Ongoing research in the use of novel agents and combinations of agents will hopefully clarify which patients and which regimens should be used in treating these patients.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Israel Deutsch, James McKiernan, Charles Drake) for the series “Prostate Cancer: Current Understanding and Future Directions” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.05.36). The series “Prostate Cancer: Current Understanding and Future Directions” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Joniau S, Van Poppel H. Localized prostate cancer: can we better define who is at risk of unfavourable outcome? BJU Int 2008;101:5-10. [Crossref] [PubMed]
- D'Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy or external beam radiation therapy for patients with clinically localized prostate carcinoma in the prostate specific antigen era. Cancer 2002;95:281-6. [Crossref] [PubMed]
- Ingimarsson JP, Celaya MO, Laviolette M, et al. Trends in initial management of prostate cancer in New Hampshire. Cancer Causes Control 2015;26:923-9. [Crossref] [PubMed]
- Weiner AB, Matulewicz RS, Schaeffer EM, et al. Contemporary management of men with high-risk localized prostate cancer in the United States. Prostate Cancer Prostatic Dis 2017;20:283-8. [Crossref] [PubMed]
- Boorjian SA, Karnes RJ, Viterbo R, et al. Long-term survival after radical prostatectomy versus external-beam radiotherapy for patients with high-risk prostate cancer. Cancer 2011;117:2883-91. [Crossref] [PubMed]
- Thompson IM, Tangen CM, Paradelo J, et al. Adjuvant radiotherapy for pathologic T3N0M0 prostate cancer. J Urol 2009;181:956-62. [Crossref] [PubMed]
- Messing EM, Manola J, Sarosdy M, et al. Immediate Hormonal Therapy Compared With Observation After Radical Prostatectomy and Pelvic Lymphadenectomy in Men With Node-Positive Prostate Cancer. N Engl J Med 1999;341:1781-8. [Crossref] [PubMed]
- Fisher B, Bryant J, Wolmark N, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol 1998;16:2672-85. [Crossref] [PubMed]
- Yin M, Joshi M, Meijer RP, et al. Neoadjuvant Chemotherapy for Muscle-Invasive Bladder Cancer: A Systematic Review and Two-Step Meta-Analysis. Oncologist 2016;21:708-15. [Crossref] [PubMed]
- Zhao B, Yerram NK, Gao T, et al. Long-term survival of patients with locally advanced prostate cancer managed with neoadjuvant docetaxel and radical prostatectomy. Urol Oncol 2015;33:164.e19-23. [Crossref] [PubMed]
- Roach M 3rd, Bae K, Speight J, et al. Short-term neoadjuvant androgen deprivation therapy and external-beam radiotherapy for locally advanced prostate cancer: long-term results of RTOG 8610. J Clin Oncol 2008;26:585-91. [Crossref] [PubMed]
- Schulman CC, Debruyne FM, Forster G, et al. 4-Year follow-up results of a European prospective randomized study on neoadjuvant hormonal therapy prior to radical prostatectomy in T2-3N0M0 prostate cancer. European Study Group on Neoadjuvant Treatment of Prostate Cancer. Eur Urol 2000;38:706-13. [Crossref] [PubMed]
- Soloway MS, Pareek K, Sharifi R, et al. Neoadjuvant androgen ablation before radical prostatectomy in cT2bNxMo prostate cancer: 5-year results. J Urol 2002;167:112-6. [Crossref] [PubMed]
- Shelley MD, Kumar S, Wilt T, et al. A systematic review and meta-analysis of randomised trials of neo-adjuvant hormone therapy for localised and locally advanced prostate carcinoma. Cancer Treat Rev 2009;35:9-17. [Crossref] [PubMed]
- Taplin ME, Montgomery B, Logothetis CJ, et al. Intense androgen-deprivation therapy with abiraterone acetate plus leuprolide acetate in patients with localized high-risk prostate cancer: results of a randomized phase II neoadjuvant study. J Clin Oncol 2014;32:3705-15. [Crossref] [PubMed]
- Efstathiou E, Davis JW, Titus MA, et al. Neoadjuvant Enzalutamide (ENZA) and Abiraterone Acetate (AA) plus Leuprolide Acetate (LHRHa) versus AA+LHRHa in Localized High-Risk Prostate Cancer (LHRPC). J Clin Oncol 2016;34:abstr 5002.
- Montgomery B, Tretiakova MS, Joshua AM, et al. Neoadjuvant Enzalutamide Prior to Prostatectomy. Clin Cancer Res 2017;23:2169-76. [Crossref] [PubMed]
- Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 2004;351:1513-20. [Crossref] [PubMed]
- Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004;351:1502-12. [Crossref] [PubMed]
- Craft N, Chhor C, Tran C, et al. Evidence for clonal outgrowth of androgen-independent prostate cancer cells from androgen-dependent tumors through a two-step process. Cancer Res 1999;59:5030-6. [PubMed]
- Eigl BJ, Eggener SE, Baybik J, et al. Timing is everything: preclinical evidence supporting simultaneous rather than sequential chemohormonal therapy for prostate cancer. Clin Cancer Res 2005;11:4905-11. [Crossref] [PubMed]
- Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 2003;349:859-66. [Crossref] [PubMed]
- Hussain M, Smith DC, El-rayes BF, et al. Neoadjuvant docetaxel and estramustine chemotherapy in high-risk/locally advanced prostate cancer. Urology 2003;61:774-80. [Crossref] [PubMed]
- Magi-Galluzzi C, Zhou M, Reuther AM, et al. Neoadjuvant docetaxel treatment for locally advanced prostate cancer: A clinicopathologic study. Cancer 2007;110:1248-54. [Crossref] [PubMed]
- Febbo PG, Richie JP, George DJ, et al. Neoadjuvant docetaxel before radical prostatectomy in patients with high-risk localized prostate cancer. Clin Cancer Res 2005;11:5233-40. [Crossref] [PubMed]
- Garzotto M, Higano CS, O'Brien C, et al. Phase 1/2 study of preoperative docetaxel and mitoxantrone for high-risk prostate cancer. Cancer 2010;116:1699-708. [Crossref] [PubMed]
- Konety BR, Eastham JA, Reuter VE, et al. Feasibility of radical prostatectomy after neoadjuvant chemohormonal therapy for patients with high risk or locally advanced prostate cancer: results of a phase I/II study. J Urol 2004;171:709-13. [Crossref] [PubMed]
- Bergstrom CP, Ruffell B, Ho CM, et al. Docetaxel and mitoxantrone before radical prostatectomy in men with high-risk prostate cancer: 10-year follow-up and immune correlates. Anticancer Drugs 2017;28:120-6. [Crossref] [PubMed]
- Clark PE, Peereboom DM, Dreicer R, et al. Phase II trial of neoadjuvant estramustine and etoposide plus radical prostatectomy for locally advanced prostate cancer. Urology 2001;57:281-5. [Crossref] [PubMed]
- Chi KN, Chin JL, Winquist E, et al. Multicenter Phase II study of combined neoadjuvant docetaxel and hormone therapy before radical prostatectomy for patients with high risk localized prostate cancer. J Urol 2008;180:565-70. [Crossref] [PubMed]
- Beltran H, Wyatt AW, Chedgy E, et al. Impact of therapy on genomics and transcriptomics in high-risk prostate cancer treated with neoadjuvant docetaxel and androgen deprivation therapy. Clin Cancer Res 2017;23:6802-11. [Crossref] [PubMed]
- Zurita AJ, Pisters LL, Wang X, et al. Integrating chemohormonal therapy and surgery in known or suspected lymph node metastatic prostate cancer. Prostate Cancer Prostatic Dis 2015;18:276-80. [Crossref] [PubMed]
- Savarese DM, Halabi S, Hars V, et al. Phase II study of docetaxel, estramustine, and low-dose hydrocortisone in men with hormone-refractory prostate cancer: a final report of CALGB 9780. Cancer and Leukemia Group B. J Clin Oncol 2001;19:2509-16. [Crossref] [PubMed]
- Fizazi K, Faivre L, Lesaunier F, et al. Androgen deprivation therapy plus docetaxel and estramustine versus androgen deprivation therapy alone for high-risk localised prostate cancer (GETUG 12): a phase 3 randomised controlled trial. Lancet Oncol 2015;16:787-94. [Crossref] [PubMed]
- Narita S, Tsuchiya N, Kumazawa T, et al. Short-term clinicopathological outcome of neoadjuvant chemohormonal therapy comprising complete androgen blockade, followed by treatment with docetaxel and estramustine phosphate before radical prostatectomy in Japanese patients with high-risk localized prostate cancer. World J Surg Oncol 2012;10:1. [Crossref] [PubMed]
- Prayer-Galetti T, Sacco E, Pagano F, et al. Long-term follow-up of a neoadjuvant chemohormonal taxane-based phase II trial before radical prostatectomy in patients with non-metastatic high-risk prostate cancer. BJU Int 2007;100:274-80. [Crossref] [PubMed]
- Silberstein JL, Poon SA, Sjoberg DD, et al. Long-term oncological outcomes of a phase II trial of neoadjuvant chemohormonal therapy followed by radical prostatectomy for patients with clinically localised, high-risk prostate cancer. BJU Int 2015;116:50-6. [Crossref] [PubMed]
- Koie T, Mitsuzuka K, Yoneyama T, et al. Neoadjuvant luteinizing-hormone-releasing hormone agonist plus low-dose estramustine phosphate improves prostate-specific antigen-free survival in high-risk prostate cancer patients: a propensity score-matched analysis. Int J Clin Oncol 2015;20:1018-25. [Crossref] [PubMed]
- Van Veldhuizen PJ, Reed G, Aggarwal A, et al. Docetaxel and ketoconazole in advanced hormone-refractory prostate carcinoma: a phase I and pharmacokinetic study. Cancer 2003;98:1855-62. [Crossref] [PubMed]
- Womble PR, Van Veldhuizen PJ, Nisbet AA, et al. A phase II clinical trial of neoadjuvant ketoconazole and docetaxel chemotherapy before radical prostatectomy in high risk patients. J Urol 2011;186:882-7. [Crossref] [PubMed]
- Thalgott M, Horn T, Heck MM, et al. Long-term results of a phase II study with neoadjuvant docetaxel chemotherapy and complete androgen blockade in locally advanced and high-risk prostate cancer. J Hematol Oncol 2014;7:20. [Crossref] [PubMed]
- Williams SB, Davis JW, Wang X, et al. Neoadjuvant Systemic Therapy Before Radical Prostatectomy in High-Risk Prostate Cancer Does Not Increase Surgical Morbidity: Contemporary Results Using the Clavien System. Clin Genitourin Cancer 2016;14:130-8. [Crossref] [PubMed]
- Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006;355:2542-50. [Crossref] [PubMed]
- Madan RA, Karzai FH, Ning YM, et al. Phase II trial of docetaxel, bevacizumab, lenalidomide and prednisone in patients with metastatic castration-resistant prostate cancer. BJU Int 2016;118:590-7. [Crossref] [PubMed]
- Ross RW, Galsky MD, Febbo P, et al. Phase 2 study of neoadjuvant docetaxel plus bevacizumab in patients with high-risk localized prostate cancer: a Prostate Cancer Clinical Trials Consortium trial. Cancer 2012;118:4777-84. [Crossref] [PubMed]
- Cha EK, Eastham JA. Chemotherapy and novel therapeutics before radical prostatectomy for high-risk clinically localized prostate cancer. Urol Oncol 2015;33:217-25. [Crossref] [PubMed]
- Vuky J, Porter C, Isacson C, et al. Phase II trial of neoadjuvant docetaxel and gefitinib followed by radical prostatectomy in patients with high-risk, locally advanced prostate cancer. Cancer 2009;115:784-91. [Crossref] [PubMed]
- Mathew P, Pisters LL, Wood CG, et al. Neoadjuvant platelet derived growth factor receptor inhibitor therapy combined with docetaxel and androgen ablation for high risk localized prostate cancer. J Urol 2009;181:81-7; discussion 87. [Crossref] [PubMed]
- Kumon H, Sasaki K, Ariyoshi Y, et al. Feasibility of Neoadjuvant Ad-REIC Gene Therapy in Patients with High-Risk Localized Prostate Cancer Undergoing Radical Prostatectomy Clin Transl Sci 2015;8:837-40. [Crossref] [PubMed]
- Kumon H, Sasaki K, Ariyoshi Y, et al. Ad-REIC Gene Therapy: Promising Results in a Patient with Metastatic CRPC Following Chemotherapy. Clin Med Insights Oncol 2015;9:31-8. [Crossref] [PubMed]


