Clinical features and prognostic factors of cryptogenic hepatocellular carcinoma
Introduction
The incidence and mortality of liver cancer rank fifth and third among all cancers (1). The common causes of liver cancer include viral hepatitis and alcoholic liver cirrhosis (1,2). The early vaccination of hepatitis B vaccine and the increased safety of blood products have significantly reduced the incidence of viral liver cancer; furthermore, alcoholic liver cirrhosis-associated liver cancer has also been controlled in recent years (3). Unfortunately, the proportions of cryptogenic hepatocellular carcinoma (cHCC), caused by non-viral hepatitis and nonalcoholic cirrhosis and with unknown etiologies, have gradually increased (4). In this article, we retrospectively analyzed the clinical data of 59 cHCC patients who had been managed in the Department of Hepatobiliary Surgery of our hospital from 1999 to 2010, with an attempt to investigate the clinical features and prognostic factors of cHCC.
Methods
Inclusion criteria
The diagnostic criteria of cHCC were as follows (5-8): (I) pathologically confirmed as primary liver cancer; (II) alcohol consumption: ≤20 g/d for men and ≤10 g/d for women; (III) both HBsAg and anti-HCV antibody were negative; and (IV) liver cancer caused by hemoglobin diseases, autoimmune hepatitis, and/or other chronic liver diseases were excluded.
Follow-up
From January 1999 to December 2010, there were 908 patients with primary hepatocellular carcinoma who underwent radical operation in our department, among whom 59 patients met the diagnostic criteria of cHCC. Patients were regularly followed up, and data including tumor recurrence and patient survival were recorded. The deadline of the follow-up was September 1, 2017.
The time interval between surgery and recurrence was defined as the disease-free survival (DFS), and the period from surgery to death/final follow-up visit as the overall survival (OS). Factors (including sex, age, BMI, ALT, AST, TBIL, DBIL, GGT, TG, CHOL, ALB, TP, blood glucose, AFP, nonalcoholic fatty liver disease (NAFLD), serological markers for hepatitis viruses, tumor size, tumor number, and pathological grade) that might affect the outcome of a cHCC surgery were analyzed.
Statistical analysis
All data were analyzed using the SPSS 22.0 software. The comparisons of the categorical variables and the continuous variables were based on χ2 test and Kruskal-Wallis test, respectively.
Survival analysis was performed by using the Kaplan–Meier method. Univariate regression analysis was carried out for the assessment of potential prognostic factors. Variables with a P value of <0.1 entered the multivariate Cox regression model. A P value of <0.05 was considered significantly different.
Results
Clinical features
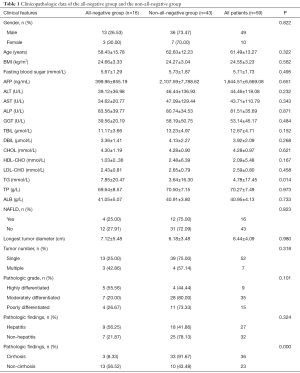
A total of 59 patients (6.49% of the 908 patients with primary liver cancer during the same period) were included in the analysis. These subjects had a mean age of 61.4 years and a BMI of (24.55±3.23) kg/m2. The male/female ratio was 4.9. Sixteen patients had NAFLD; the hepatitis virus serologic markers were all negative in 16 patients (all-negative group) but were not all negative in the remaining 43 patients (non-all-negative group, with HBcAb expression in each subject). Detection of tumor markers showed AFP ≥7 ng/mL in 23 cases (38.98%), whereas CEA was normal in all patients.
Liver function tests showed: ALT >50 U/L in 13 cases (22.3%); AST >40 U/L in 10 cases (16.95%); TBil >17.1 µmol/L in 10 cases (16.95%); and TG >1.69 mmol/L in 10 cases (16.95%). The clinical data of these 59 patients are summarized in Table 1.
Full table
Imaging examinations
All 59 patients underwent CT and ultrasonography, and 40 patients received magnetic resonance imaging (MRI). The imaging data showed that 16 patients (27.12%) were suffering from fatty liver.
Clinical treatment and prognosis
All the 59 patients underwent radical surgical treatment, among whom 17 also received postoperative interventional therapy, radiotherapy, chemotherapy, radiofrequency (RF) ablation, and other treatments. The median follow-up duration for these 59 cHCC patients was 32 months, and the post-operative 1-, 3-, and 5-year survival rates were 81.36%, 44.07%, and 35.59%, respectively; the longest DFS was 195 months, and the median survival time reached 32 months.
Post-operative pathology
HCC was confirmed after postoperative pathologic examinations in all these 59 patients.
According to the 2010 WHO Classification of Digestive Tumors, the tumors were highly differentiated in 9 cases, moderately differentiated in 35 cases, and poorly differentiated in 15 cases.
There were 14 cases of liver cirrhosis complicated with hepatitis and 10 cases without any hepatitis or liver cirrhosis. The tumors were single in 52 cases and multiple in 7 cases. No metastasis was noted in all 59 cases. The tumor size ranged 1.7–18 cm. The distribution of hepatitis virus serologic markers significantly differed between all-negative group and non-all-negative group (P=0.000) (Table 1).
Influences of clinical indicators on the postoperative OS
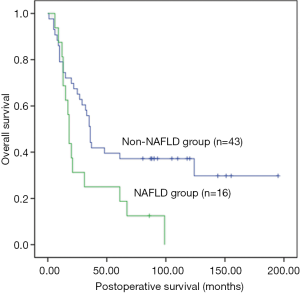
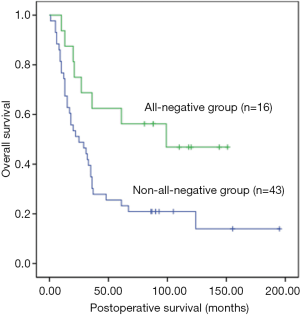
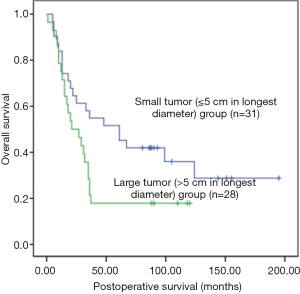
Univariate analysis showed that the postoperative survival was significantly superior in the all-negative group (n=16) than in non-all-negative group (χ2=5.918, P=0.015) (Figure 1), whereas that in the non-NAFLD group (n=43) was significantly longer than in NAFLD group (χ2=5.401, P=0.02) (Figure 2). The survival time of small tumor (≤5 cm in longest diameter) group was longer than that of large tumor (>5 cm in longest diameter) group (P=0.045) (Figure 3). Variables with a P value of <0.1 entered the multivariate Cox regression model. Multivariate analysis showed that positive HBcAb expression (HR =2.558, 95% CI: 1.146–5.711, P=0.022), NAFLD (HR =2.067, 95% CI: 1.056–4.049, P=0.034), and large tumor (>5 cm in longest diameter) (HR =2.190, 95% CI: 1.155–4.154, P=0.016) were the independent factors that affected the OS (Table 2).
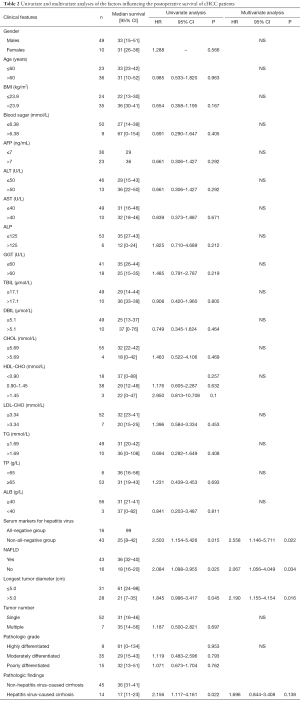
Full table
Discussion
The concept of cryptogenic liver cirrhosis was proposed by Stephen et al. in 2004 (9). On this basis, some authors further described the definition of cHCC (5-8). “Cryptogenic” does not mean unknown etiology. Rather, cHCC specifically refers to liver cancers that are not caused by hepatitis B, alcoholic cirrhosis, and other specific pathogenic factors but caused by relatively obscure etiologies (7,8).
It has been reported that NAFLD and other metabolic syndromes are closely related to the occurrence of cHCC (10). With the change of lifestyle and the increase of obese populations, the proportion of cHCC associated with NAFLD and metabolic liver diseases are increasing (4,11). In Europe and North America, the number of cHCC patients has shown an increasing trend (8,12). In China, however, few literature has described such primary hepatocellular carcinoma.
cHCC is a relatively rare disease and occurs mainly in elderly obese males (Table 3). As shown in our current study, cHCC cases accounted for 6.49% of the primary liver cancer patients that were treated during the same period. These cHCC patients had a mean age of 61 years and a BMI of (24.55±3.23) kg/m2. The male/female ratio was 4.9. These findings were consistent with the above conclusions. HW Kwak et al. have demonstrated that HBcAb-positive cHCC accounted for about 84.6% of all cHCC cases in hepatitis B virus endemic area (8). A prospective cohort study indicated that HBsAg would be cleared with age in patients with hepatitis B virus infection, along with HbcAb expression; in some patients, liver cancer occurred within 7.3 years after the removal of HBsAg (13).
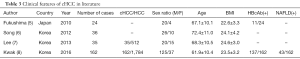
Full table
These phenomena may be explained by the fact that the HBsAg clearance is related only to the increasing age. HBsAg removal often takes a long period of time; therefore these patients have longer exposure to the destructive effect caused by HBV DNA integration, and such destructive effect is reflected in the following mechanism: by integrating into hepatocyte genome, the HBV DNA causes long-term inflammation and human hepatocyte genome mutation, and their persisting effects lead to the occurrence of liver cancer (14). Thus, these HBcAb-positive cHCC occurred actually following hepatitis B virus infection accompanied by HBsAg clearance and HBV DNA integration. It has been reported that more than half of the cHCC cases in non-viral hepatitis endemic countries are NAFLD-related cHCC (10,15,16). NAFLD is a group of diseases that are radiologically or histologically manifested as the fatty change of hepatocyte and are not caused by excessive alcohol use, drugs, or hereditary diseases (17). The pathogenic mechanism of NAFLD-associated cHCC is as follows: excessive deposition of liver fat caused by obesity and drugs may induce the increase of TNFα and IL-6 and thus cause inflammation in liver, eventually leading to the occurrence of liver cancer (18). In our current series, HBcAb-positive cHCC accounted for 72.88% and NAFLD patients accounted for 27.12%. China has a high prevalence of hepatitis virus infection, along with a large proportion of hepatitis patients. The proportion of samples in this study is consistent with our national conditions.
Few literatures have described the prognosis of patients with cHCC after surgery. In our current series, the post-operative 3-, and 5-year survival rates were 44.07% and 35.59%, respectively; the longest DFS was 195 months, and the median survival time reached 32 months. Whether cHCC has a better prognosis than non-cHCC remains controversial. In HW Kwak et al.’s study (8), the prognosis of cHCC was superior to that of virus-associated liver cancer; however, Sang SL’s study has demonstrated that the prognosis of cHCC was not significantly different from those of viral HCC and alcoholic liver cancer (6).
Up to now no study has investigated the prognostic factors of cHCC. In our current study, univariate analysis showed that the median survival time of HBcAb-positive group, NAFLD group, liver cirrhosis accompanied with hepatitis group, and large tumor size (>5 cm) group was significantly shorter than their counterparts. Multivariate analysis showed that HBcAb (+), NAFLD, and large tumor size (>5 cm) were independent prognostic factors for the postoperative survival of cHCC patients. Clinically HBcAb (+) status and NAFLD can be used as important prognostic predictors for cHCC patients after surgery. Compared with the HBsAg (+) status and alcoholic fatty liver, HBSAG (−) HBcAb (+) status and NAFLD are often neglected and their treatments are delayed due to the lack of specific clinical features.
In summary, cHCC is a relatively rare disease and occurs mainly in elderly obese males. The development of cHCC is associated with NAFLD and hepatitis B virus Infection. HBcAb (+) cHCC is the predominant type in hepatitis B virus endemic area. HBcAb (+) and NAFLD are independent prognostic factors for the postoperative survival of cHCC patients. However, our current study was limited by its single-center retrospective design and small sample size, and it conclusions need to be confirmed by more randomized controlled studies with larger sample sizes.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.06.06). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee board of National Cancer Center/Cancer Hospital (No. 17-194/1450) and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Xie DY, Ren ZG, Zhou J, et al. Critical appraisal of Chinese 2017 guideline on the management of hepatocellular carcinoma. Hepatobiliary Surg Nutr 2017;6:387-96. [Crossref] [PubMed]
- Tang H, Huang Y, Duan W, et al. A concise review of current guidelines for the clinical management of hepatocellular carcinoma in Asia. Transl Cancer Res 2017;6:1214-25. [Crossref]
- Tateishi R, Okanoue T, Fujiwara N, et al. Clinical characteristics, treatment, and prognosis of non-B, non-C hepatocellular carcinoma: a large retrospective multicenter cohort study. J Gastroenterol 2015;50:350-60. [Crossref] [PubMed]
- Yang JD, Roberts LR. Hepatocellular carcinoma: A global view. Nat Rev Gastroenterol Hepatol 2010;7:448-58. [Crossref] [PubMed]
- Fukushima N, Kuromatsu R, Akiba J, et al. Characteristic expression pattern of oxidative stress in livers with cryptogenic hepatocellular carcinoma. Exp Ther Med 2010;1:809-16. [Crossref] [PubMed]
- Song HY, Keun LH, Sung LJ, et al. Risk factors of cryptogenic hepatocellular carcinoma in patients with low body mass index or without metabolic syndrome. Korean J Intern Med 2012;27:47-52. [Crossref] [PubMed]
- Lee SS, Jeong SH, Byoun YS, et al. Clinical features and outcome of cryptogenic hepatocellular carcinoma compared to those of viral and alcoholic hepatocellular carcinoma. BMC Cancer 2013;13:335. [Crossref] [PubMed]
- Kwak HW, Park JW, Koh YH, et al. Clinical Characteristics of Patients with Cryptogenic Hepatocellular Carcinoma in a Hepatitis B Virus-Endemic Area. Liver Cancer 2016;5:21-36. [Crossref] [PubMed]
- Caldwell SH, Crespo DM. The spectrum expanded: cryptogenic cirrhosis and the natural history of non-alcoholic fatty liver disease. J Hepatol 2004;40:578-84. [Crossref] [PubMed]
- Adams LA, Lindor KD. Nonalcoholic fatty liver disease. Ann Epidemiol 2007;17:863-9. [Crossref] [PubMed]
- Romero-Gómez M, Zelber-Sagi S, Trenell M. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol 2017;67:829-46. [Crossref] [PubMed]
- Siegel AB, Zhu AX. Metabolic syndrome and hepatocellular carcinoma: two growing epidemics with a potential link. Cancer 2009;115:5651-61. [Crossref] [PubMed]
- Simonetti J, Bulkow L, McMahon BJ, et al. Clearance of hepatitis B surface antigen and risk of hepatocellular carcinoma in a cohort chronically infected with hepatitis B virus. Hepatology 2010;51:1531-7. [Crossref] [PubMed]
- Ringelhan M, Heikenwalder M, Protzer U. Direct effects of hepatitis B virus-encoded proteins and chronic infection in liver cancer development. Dig Dis 2013;31:138-51. [Crossref] [PubMed]
- Marrero JA, Fontana RJ, Su GL, et al. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology 2002;36:1349-54. [Crossref] [PubMed]
- Hadjittofi C, Athanasopoulos PG, Koti RS, et al. Long-term survival with repeated resections of recurrent hepatocellular carcinoma in a non-cirrhotic liver: case report and brief review of the literature. Ann Transl Med 2016;4:112. [Crossref] [PubMed]
- Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology 2012;142:1592-609. [Crossref] [PubMed]
- Alzahrani B, Iseli TJ, Hebbard LW. Non-viral causes of liver cancer: does obesity led inflammation play a role? Cancer Lett 2014;345:223-9. [Crossref] [PubMed]