
Diagnosis and treatment of primary hepatic lymphoma
Introduction
Primary hepatic lymphoma (PHL) is a rare type of non-Hodgkin’s lymphoma, which accounts for approximately 0.4% of extranodal non-Hodgkin’s lymphoma and 0.016% of all non-Hodgkin’s lymphoma (1). There are a few single cases reported before, but no significant features have been concluded due to the small-scale research. Most PHL is believed to originate from B cell. T-cell lymphoma in liver is even fewer and only a few cases of that have been reported so far (2).
PHL usually presents discomfort or pain in the right upper quadrant of the abdomen and B symptoms (fever, night sweats, weight loss) as well. Anorexia, nausea and vomiting are accompanied sometimes. The most common sign in physical and imaging examination is hepatomegaly. Splenomegaly and cholestatic jaundice are also reported in some cases. Computed tomography (CT) and ultrasound are performed in every patient, which are recommended in the differential diagnosis of focal hepatic lesions (3). The typical radiological appearance could be single solitary or multiple lesions and diffuse infiltration of the liver could also be observed in some cases.
As PHL is a rare disease without specific clinical symptoms and imaging performance, it can be easily confused with hepatitis, benign and malignant liver masses (4). Therefore, in this study, we collected nine pathologically diagnosed PHL cases and fully described their clinical manifestations, pathologic features, imaging characteristics, treatment schema and outcome. Besides, almost half of the cases collected in this study are T-cell lymphoma, which is different from the B cell-originated PHL in most case reports (2,5).
Methods
The clinical records of patients with liver lymphoma from Mar 1st, 2000 to Nov 30th, 2016 in the single center-Peking Union Medical College Hospital (PUMCH) were reviewed. The patient inclusion criteria were as follows (6): (I) the symptoms and physical signs caused by liver have already presented at the time of diagnosis; (2) there are no evidence showing extrahepatic tissue, such as lymph nodes, bone marrow and peripheral blood are involved; (III) lymphomatous infiltration of the spleen and lymph nodes in the hepatic hilum at time of diagnosis were not exclusive. Eventually, based on the criteria listed, nine patients were qualified and included into the study.
Several laboratory tests were completed, including liver function test, HIV and hepatitis viruses infection state, tumor markers as well as some inflammatory markers (C-reactive protein, erythrocyte sedimentation rate), which were all obtained at the first time of the visit. Abdominal ultrasound and CT were performed in all the patients, while the magnetic resonance imaging (MRI), whole body scan and positron emission tomography (PET) were not available for most of the patients. Percutaneous liver biopsies under CT guidance were performed in six patients and the other three underwent surgical procedure and got the pathological results. All patients received a bone marrow aspiration and biopsy and peripheral blood smear as part of the routine procedures.
The protocol of this study was approved by the institutional ethical committee of PUMCH with No. 2017-pumch-030. The written informed consents were obtained from every patient when they were received treatment in PUMCH.
Results
Clinical manifestation
Among all the cases, six were female and three were male, the age range of which was from 12 to 66 years (mean 36.1 years). The main clinical manifestations were summarized in Table 1 and all the patients had no relevant clinical history. The main symptoms of our cases included pain (3#, 4#, 5#, 6#, 8#, 9#) and B symptoms (fever, night sweats, weight loss) (2#, 4#, 5#, 7#). Right upper quadrant epigastric discomfort was complained by one patient (1#). Two patients presented digestive symptoms: diarrhea (1#), nausea (4#) and vomiting (4#). Another patient who had a prolonged activated partial thromboplastin time (ATPP) also complained hemorrhinia (5#).
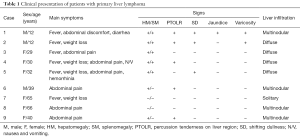
Full table
Physical examination revealed hepatosplenomegaly in five patients (1#, 2#, 3#, 4#, 5#), among which, percussion tenderness on liver region turned positive in three (1#, 2#, 4#) and shifting dullness positive in three (1#, 2#, 5#). Besides that, two showed varicosities on presentation (2#, 5#) and one had jaundice (1#). The liver was enlarged in another two patients (6#, 9#) who were not accompanied by splenomegaly. The remaining two patients (7#, 8#) showed no obvious abnormalities.
Auxiliary examinations
The biochemical test results were summarized in Table 2. Raised serum liver enzymes level were the most constantly recorded laboratory abnormalities, especially the alkaline phosphatase, aminotransferase and lactate dehydrogenase. The liver function tests of all the patients presented abnormal and two of them had a prolonged APTT (2#, 5#). Inflammatory markers were raised in three patients (1#, 3#, 7#). The serum α-fetoprotein (AFP) was normal in nine patients. Three patients had an increased serum CA-199 (6#, 7#, 8#), which was highly significant in the diagnosis of pancreatic carcinoma and cholangiocarcinoma.

Full table
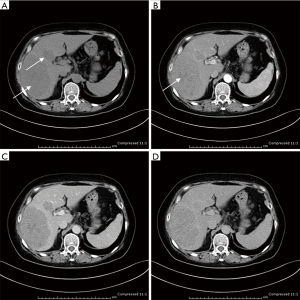
Ultrasonogram and computed tomography of abdomen were performed in all patients, which demonstrated multiple nodules in liver in four patients (1#, 6#, 8#, 9#), solitary liver infiltration in only one patients (7#) and diffuse hepatomegaly in the rest. It is interesting that most lesions in liver showed hypoechoic in ultrasound and hypoattenuating in CT (Figure 1), except for patient 2. Hepatosplenomegaly were presented in seven patients, consistent with the physical examination. In four patients (1#, 2#, 3#, 5#), lymph node enlargement of the hepatic hilum was revealed.
Bone marrow aspirates and peripheral blood smear, done in all nine cases, showed no evidence of lymphoma. Three splenectomies (2#, 3#, 4#) combined with liver biopsy, and six percutaneous liver biopsies were performed. Based on the combination of imaging and biopsy results, the macroscopic feature of the tumors could be described as diffuse, multinodular or solitary nodules.
Pathological features
The pathologic and immunofluorescence results were summarized in Table 3.
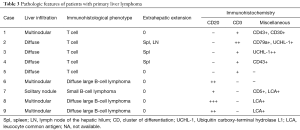
Full table
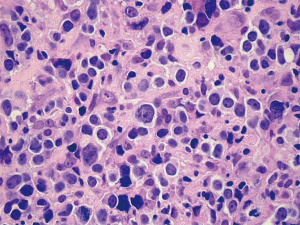
Among patients diagnosed with T-cell lymphoma, tumor cells were all CD3 positive and CD20 negative. Based on the NCCN (National Comprehensive Cancer Network) clinical practice guidelines about non-Hodgkin’s lymphoma, we couldn’t make a immunohistological diagnosis of these T-cell lymphomas due to lack of enough evidence. Patient 7 had an unusual small B-lymphocytic lymphoma, while patient 6, 8 and 9 had a diffuse large B-cell lymphoma (Figure 2). Lymphoma cells of these cases were all positive for CD20 and negative for CD3.
Definite extrahepatic extension was demonstrated by pathological analysis after surgery in three patients (2#, 3#, 4#). The other patients received the percutaneous liver biopsies only showed no evidence of extrahepatic extension.
Diagnosis and differential diagnosis
Only based on the clinical manifestations and imaging results, PHL could not be diagnosed. The definite diagnosis was established only when the immunohistochemical result was acquired and the evidence proving that no lymphoproliferative disease existed outside the liver was found. According to the PHL diagnosis criteria proposed by Lei (6), the clinical and biological features could be suggestive for PHL diagnosis, but the liver biopsy was the only way to confirm that. Some other studies recommended using the changing level of serum lactate dehydrogenase (LDH) as a diagnostic marker (7,8). However, due to its poor specificity, the value of that was limited.
In other studies, some cases who presented a solitary mass, multiple lesions or diffuse hepatic involvement in radiology resembled the primary liver tumor or the metastatic liver tumor (9). Therefore, the differential diagnosis of patients with hepatic mass should take PHL into account.
Treatments and outcome
The final diagnosis, treatment regimen and eventual outcome of the patients were summarized in Table 4. As this is a retrospective study, three patients (1#, 4#, 5#) who gave up treatment after diagnosis were lost to follow and were excluded from the outcome analysis. The median survival of the rest was 21.3 months (range from 1.5 to 61 months after symptom presentation).
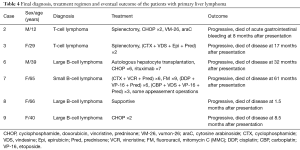
Full table
Most of the liver lesions in our patients were diffuse or multinodular involvement and this was one of the main reasons that no liver mass resection was performed. Splenectomy was undergoing in two patients (2#, 3#) and lymphadenopathy was noted, but resection was performed only in Patient2 during the surgery. Both of them received combination chemotherapy after the surgery. Adjuvant combination chemotherapy including cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP) was initiated in patient 2 after the diagnosis of T-cell lymphoma. The chemotherapy regimen was then turned to vumon-26 and cytosine arabinoside because the former one showed no effects. Unfortunately, she failed to respond either and died of acute gastrointestinal bleeding 8 months later. Patient 3 demonstrated a good partial response to cyclophosphamide, vindesine, epirubicin and prednisone, resulting in normalization of liver function tests, but she gave up the treatment for economic reason and died of the disease 17 months later.
Patient 8 was elderly and had a poor health status due to hypercalcemic crisis. She only received a series of supportive treatment. Her condition got worse and worse rapidly with disease progressing and she died at 1.5 months after the presentation. The cause of her death was acute respiratory and circulatory failure.
Patient 9 was the only one who just received CHOP after the diagnosis. However, the therapeutic effect was not satisfied and her condition kept progressed. She died of the disease at 8.5 months after the presentation.
The other two patients received chemotherapy. An autologous hepatocyte transplantation was performed in Patient6 but failed. Then a chimeric monoclonal antibody named rituximab, which could bind to the CD20 on the cell surface, was used in the treatment after he had poor response to CHOP. Rituximab worked at first, but the disease progressed later and he died of the uncontrollable disease eventually. Patient 7 had hepatomegaly when first diagnosed and the liver markedly shrunk by 41% comparing with the computed tomography results at the beginning of the treatment with cytosine arabinoside, vincristine and prednisone. But as the disease progressed, the treatment was changed to a series of other combination chemotherapy listed in Table 4 and she died of multiple organs involvement (lung, bone, etc.) at 61 months after the presentation. During the treatment, she also had some appeasement operations to alleviate the symptoms.
In summary, our series had nine patients, whose median age was 36.1 years old and had a female predominance (66.7%). Abdominal pain or uncomfortable (77.8%), B symptoms, which included fever (66.7%) and weight loss (44.4%), were the most frequent two features. Hepatomegaly was found in seven patients (77.8%) and splenomegaly in five (55.6%). None of them had preexisting liver disease. Physical examination mainly revealed positive percussion tenderness in liver region in five (55.6%). The biochemical presentation indicated striking dysfunction of liver (100%). Four patients had diffuse liver infiltration. Four had multiple nodules and only one had solitary nodular. Immunohistologic subtypes were consisted of T-cell lymphoma (55.6%), diffuse large B-cell lymphoma (6#, 8#, 9#) and unusual small B-lymphocytic lymphoma (7#). Among the nine patients, three were lost to follow-up. In the rest of the patients, two received splenectomy. One performed autologous hepatocyte transplantation and all received combination chemotherapy. The median survival of them was 21.3 months (range, 1.5–61 months).
Discussion
According to the previous literature, PHL could occur at any age but mainly involve middle-aged women (6). However, in our series, there were two 12-year-old pediatric patients making the median age of the cases locate in the thirties, which is quite usual (10). Patients usually presented with abdominal pain and/or hepatomegaly and sometimes might be accompanied with fulminant hepatic failure. The liver mass could be solitary or multiple in most cases (6,11). The B symptoms (i.e., fever, night sweats or weight loss) were as frequent as abdominal pain in our patients and different from what had been reported in the literature. Most of our patients showed diffuse liver lesions in the ultrasonogram and CT scan. It was reported that liver lymphomatous infiltration might be associated with liver function alteration even the AFP level was in the normal range (12), which was observed in our cases. Besides that, we had noticed that the raised serum liver enzymes activities were the most striking recorded laboratory abnormalities, including alkaline phosphatase, lactate dehydrogenase and aminotransferase. None of our patients had positive hepatitis virus surface antigen or relative liver disease before. According to a former research, some autoimmune diseases and acquired immune deficiency syndrome could contribute to the development of PHL (13), which didn’t happen in our study.
Similar to the previous researches, the clinical presentation was far from being specified, because some other hepatic diseases, such as hepatocellular carcinoma, could present in a semblable manner (14). Moreover, these diseases were much more common all over the world, especially in China. It was also believed that biochemistries and radiologic assessment were not decisive in the diagnosis, although normal serum AFP might be helpful in the differential diagnosis of hepatocellular carcinoma. Nevertheless, recent studies indicated some general features in imaging, which were observed in our study as well, that abdominal ultrasound commonly revealed hypoechoic lesions and CT scan usually revealed hypoattenuating. After the administration of an intravenous contrast, only a few of them showed patchy enhancement or a ring-pattern enhancement and the others were not enhanced at all. Generally, PHL were hypointense or isointense on T1-weighted MRI and hyperintense on Tw-weighted images. If diffusion weighted imaging (DWI) was additionally performed, the lesions showed lower ADC values (2,6,15-17). High level of fluorodeoxyglucose (FDG) uptake could help with the differentiation as well (2,18). Therefore, we suggested that a combination of image modalities be used in the diagnosis.
As we all know, a definite diagnosis of PHL could only be acquired after the pathological assessment of liver biopsy. Researchers suggested that patients who showed hepatomegaly and systemic symptoms with unknown causes should undergo a liver biopsy as early as possible because specific treatment could be performed timely before liver failure and/or lymphoma dissemination (11). It was demonstrated in a previous research that it was a safe and effective choice to perform the image-guided liver biopsy in PHL patients when there was no alternative tissue to choose (6). In our series, percutaneous liver biopsies under CT guidance were performed in six immediately after catching the sign of hepatomegaly, contributing a lot to the diagnosis. The other three underwent liver biopsy by surgery. The pathological analysis of PHL in literatures manifested that diffuse large cell lymphoma was the predominant histologic subtype (19,20) and most cases were originated from B-cell lineage which were confirmed by immunohistochemical studies (2). In contrast, only three of nine patients in our series had diffuse large cell lymphomas and five of nine originated from T-cell. Recent studies also showed some interesting results. For example, some indicated that the nodular liver infiltration was corresponded to high grade B-cell lymphoma, while the diffuse liver infiltration was related to either T-cell or B-cell lymphomas (11). As in our series, four of five nodular involvements were B-cell lymphomas, three diffused large B-cell lymphomas and one small B-cell lymphoma. All diffuse liver involvements were T-cell subtype. We inferred that the relatively high ratio of T-cell lymphomas in our cases may attribute to the high incidence of T-cell lymphomas in our living region.
As our study was a retrospective study, it was impossible to conduct a survival analysis for all patients. Therefore, we compared the research findings of other published articles with our study. The previous studies regarded the primary liver lymphoma as a highly aggressive disease with a relatively poor outcome, especially in China (1,2,6,15,21). The poor prognosis was attributed to advanced age, bulky disease, constitutional symptoms, comorbid conditions as well as unfavorable histologic subtypes (2). It had been reported that the patients who had nodular liver infiltration showed a significantly better prognosis than those who had diffuse infiltration (P=0.0033). Additionally, when treated with a chemotherapy regimen containing anthracycline, patients who had nodular primary liver NHL showed a better prognosis (P<0.0001) (8). The poor median survival of our patients (21.3 months) could be explained partly by the fact that most of our patients had diffuse liver lesions and the surgical resection thus could not be performed to them except for patient 7, who got some appeasement operations and survived almost 5.1 years. Although the outcome of PHL was generally poor, there were still some single case reports showing a favorable outcome (22-25) reported that a low-dose corticosteroid, the THP-COP (therarubicin, cyclophosphamide, vincristine) protocol or the addition of rituximab could improve the prognosis of those NHL resistant to current treatments.
Conclusions
Patients who had primary liver lymphoma tended to present some non-specific clinical, serological and radiological features. Abdominal pain, B symptoms and hepatosplenomegaly were three most frequently presenting features. The tumor markers in these patients were normal, which is different from the most cases of hepatocellular carcinoma (26). The liver function was compromised in most cases. The imaging features of patients revealed solitary, multiple and diffuse lesions, which were hypoechoic in ultrasounds and hypoattenuating in CT. The median survival time was about 21.3 months.
In a word, PHL is an aggressive disease and the best treatment still remains uncertain with few patients achieved complete remission or survived. As the number of case reports of PHL keep growing and bad prognosis of some patients partly related to the diagnostic delay, it’s necessary for clinicians to pay attention to the patients with the common clinical and imaging features. In this way, we may be able to diagnose the disease early by using the method of liver biopsy and have more alternative treatment choices for the patients.
Acknowledgments
Dr. Ji Li from the Pathological Department of Peking Union Medical College provided the technical help. Picture 2 was provided by him and we appreciate his participation and kind help sincerely.
Funding: This work was supported by Youth Scientific Research Fund of Peking Union Medical College Hospital (pumch-2013-045).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.06.03). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethical committee of PUMCH (No. 2017-pumch-030) and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Freeman C, Berg JW, Cutler SJ. Occurrence and prognosis of extranodal lymphomas. Cancer 1972;29:252-60. [Crossref] [PubMed]
- Noronha V, Shafi NQ, Obando JA, et al. Primary non-Hodgkin’s lymphoma of the liver. Crit Rev Oncol Hematol 2005;53:199-207. [Crossref] [PubMed]
- Xie DY, Ren ZG, Zhou J, et al. Critical appraisal of Chinese 2017 guideline on the management of hepatocellular carcinoma. Hepatobiliary Surg Nutr 2017;6:387-96. [Crossref] [PubMed]
- Chi T, Lu X. Hepatocellular carcinoma: an entity needed to be differentiated. Hepatobiliary Surg Nutr 2016;5:451-2. [Crossref] [PubMed]
- Jaffe ES. Malignant lymphomas: pathology of hepatic involvement. Semin Liver Dis 1987;7:257-68. [Crossref] [PubMed]
- Lei KI. Primary non-Hodgkin’s lymphoma of the liver. Leuk Lymphoma 1998;29:293-9. [Crossref] [PubMed]
- Page RD, Romaguera JE, Osborne B, et al. Primary hepatic lymphoma: favorable outcome after combination chemotherapy. Cancer 2001;92:2023-9. [Crossref] [PubMed]
- Taketomi A, Takenaka K, Shirabe K, et al. Surgically resected primary malignant lymphoma of the liver. Hepatogastroenterology 1996;43:651-7. [PubMed]
- Kaneko K, Nishie A, Arima F, et al. A case of diffuse-type primary hepatic lymphoma mimicking diffuse hepatocellular carcinoma. Ann Nucl Med 2011;25:303-7. [Crossref] [PubMed]
- Citak EC, Sari I, Demirci M, et al. Primary hepatic Burkitt lymphoma in a child and review of literature. J Pediatr Hematol Oncol 2011;33:e368-71. [Crossref] [PubMed]
- Emile JF, Azoulay D, Gornet JM, et al. Primary non-Hodgkin’s lymphomas of the liver with nodular and diffuse infiltration patterns have different prognoses. Ann Oncol 2001;12:1005-10. [Crossref] [PubMed]
- Sadahira Y, Ohmoto K, Yamamoto S, et al. Expression of cytotoxic molecule TIA-1 in malignant lymphomas mimicking fulminant hepatitis. Pathol Int 1998;48:695-704. [Crossref] [PubMed]
- Caccamo D, Pervez NK, Marchevsky A. Primary lymphoma of liver in the acquired immunodeficiency syndrome. Arch Pathol Lab Med 1986;110:553-5. [PubMed]
- Shiu W, Dewar G, Leung N, et al. Hepatocellular carcinoma in Hong Kong: clinical study on 340 cases. Oncology 1990;47:241-5. [Crossref] [PubMed]
- Salmon JS, Thomson MA, Arildsen RC, et al. Non-Hodgkin lymphoma involving the liver: clinical and therapeutic considerations. Clin Lymphoma Myeloma 2006;6:273-80. [Crossref] [PubMed]
- Maher MM, McDermott R, Fenlon HM, et al. Imaging of primary non-Hodgkin’s lymphoma of the liver. Clin Radiol 2001;56:295-301. [Crossref] [PubMed]
- Koike N, Cho A, Nasu K, et al. Role of diffusion-weighted magnetic resonance imaging in the differential diagnosis of focal hepatic lesions. World J Gastroenterol 2009;15:5805-12. [Crossref] [PubMed]
- Chen HW, Sheu JC, Lin WC, et al. Primary Liver Lymphoma in a Patient with Chronic Hepatitis C. J Formos Med Assoc 2006;105:242-6. [Crossref] [PubMed]
- Aozasa K, Mishima K, Ohsawa M. Primary malignant lymphoma of the liver. Leuk Lymphoma 1993;10:353-7. [Crossref] [PubMed]
- Ohsawa M, Aozasa K, Horiuchi K, et al. Malignant lymphoma of the liver. Report of five cases and review of the literature. Dig Dis Sci 1992;37:1105-9. [Crossref] [PubMed]
- Lei KI, Chow JH, Johnson PJ. Aggressive primary hepatic lymphoma in Chinese patients. Presentation, pathologic features, and outcome. Cancer 1995;76:1336-43. [Crossref] [PubMed]
- Miyashita K, Tomita N, Oshiro H, et al. Primary hepatic peripheral T-cell lymphoma treated with corticosteroid. Intern Med 2011;50:617-20. [Crossref] [PubMed]
- Serrano-Navarro I, Rodríguez-López JF, Navas-Espejo R, et al. Primary hepatic lymphoma - favorable outcome with chemotherapy plus rituximab. Rev Esp Enferm Dig 2008;100:724-8. [PubMed]
- Ma YJ, Chen EQ, Chen XB, et al. Primary hepatic diffuse large B cell lymphoma: A case report. Hepat Mon 2011;11:203-5. [PubMed]
- Takauchi K, Kono H, Ito T, et al. A case of primary hepatic lymphoma successfully treated by THP-COP therapy. Nihon Ronen Igakkai Zasshi 2003;40:65-8. [Crossref] [PubMed]
- Xu EC. Prof. Wei Tang-biomarkers: evaluation of early diagnosis and prognosis for patients with hepatocellular carcinoma. Hepatobiliary Surg Nutr 2018;7:158-60. [Crossref] [PubMed]


