
Upregulation of exosomal integrin β4 causes osteosarcoma cell proliferation via the PI3K-Akt-mTOR signaling pathway
Introduction
Osteosarcoma is the most common bone cancer and is the second-leading cause of cancer deaths among adolescents and young adults (1,2). Although there have been many advances in the diagnosis and treatment strategies for osteosarcoma (patients now have a higher survival rate, at 60–80%), the prognosis remains unsatisfactory, especially for patients at advanced stages (3). Thus, further elucidation of the pathogenesis of osteosarcoma is urgently needed to improve the rates of the diagnosis, therapy, and prognosis of osteosarcoma patients.
Integrins belong to the family of heterodimeric transmembrane cell surface receptors, which contain α and β subunits that conjointly connect the cytoskeleton to elements in the extracellular matrix or to adjacent cells (4). The α6β4 integrin (referred to as integrin β4) is an adhesion receptor for the laminins that performs a crucial function both in normal tissue development and cell homeostasis as well as in carcinoma progression (5). Integrin β4 has been reported to play a pivotal role in progression of many types of tumors, including osteosarcoma, prostate cancer, breast cancer, and ovarian cancer in a ligand-independent manner to promote proliferation, migration, and invasion (6-10). A recent report showed that exosomal integrins may predict organ-specific metastasis, and exosomal integrin β4 is associated with lung metastasis (7). Thus, it can be said that exosomal integrin β4 is involved in carcinoma progression; however, its role in osteosarcoma remains elusive. Taken together, these reasons inspired us to further explore the usefulness of exosomal integrin β4 for predicting the risk of osteosarcoma progression.
Accumulating evidence has suggested that PI3K-Akt-mTOR signaling is involved in the progression of many types of tumors including osteosarcoma (11,12). PI3K-Akt-mTOR signaling practically takes part in the whole progression of a tumor: from tumorigenesis, cancer cell proliferation, metastasis, and angiogenesis to chemoresistance (13). A previous work indicated that integrin β4 cooperates with the PI3K-Akt-mTOR signaling pathway to promote invasive growth and metastasis of breast cancer cells (14). These clues prompted us to investigate the interaction between integrin β4 and PI3K-Akt-mTOR signaling in osteosarcoma.
The present study was aimed at investigating the role of integrin β4 in the pathogenesis of osteosarcoma as well as the underlying molecular mechanism in enhancing osteosarcoma cell proliferation with regard to regulation of the PI3K-Akt-mTOR signaling pathway.
Methods
Patients and blood sample collection
Blood samples were obtained from 46 patients with a diagnosis of osteosarcoma and 38 healthy donors in the Xijing Hospital, The Fourth Military Medical University, from June 2014 to October 2016. All the samples from patients were collected immediately after diagnosis, without any treatment. Written consent for participation in the study was obtained from all the participants or, where participants were children, from a parent or guardian. This study protocol was approved by the ethics committee of the Xijing Hospital, The Fourth Military Medical University (approval No. ChiCTR-OPC-17010758). Patients’ clinical information is showed in Table 1.

Full table
Cell culture and treatment methods
MG-63 and U2-OS cells were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China) and cultured in the DMEM medium with 10% of fetal bovine serum (FBS, Gibco, USA) in a humidified atmosphere containing 5% of CO2 and 95% of air at 37 °C. For PI3K inhibitor LY294002 treatment experiments, the cells in culture medium were incubated with or without LY294002. Cells where the final concentration of LY294002 reached 20 mmol/L were regarded as a treatment group, and the cells incubated without LY294002 served as the control group. All unmentioned chemical reagents used in this study were purchased from Sigma-Aldrich (USA).
Enzyme-linked immunosorbent assay (ELISA)
Integrin β4 expression in peripheral blood or in the cell culture medium was measured via an ELISA kit (Sigma, RAB0212). The absorbance was detected by Thermo Multiskan Ascent at 450 nm (Thermo, USA). Each experiment was performed three times.
Isolation of exosomes by differential centrifugation
Exosomes were isolated from a cell culture medium or serum by differential centrifugation on Eppendorf Centrifuge 5804 R. Serum and cells were centrifuged at 10,000 ×g for 30 min at 4 °C in a A-4–44 swinging-bucket rotor, and the supernatant was centrifuged at 10,000 ×g for 30 min at 4 °C in the identical rotor to remove shed vesicles and other vesicles of bigger sizes, and then centrifuged at 100,000 ×g for 60 min at 4 °C in a Type 60 Ti rotor to obtain purified exosomes. Finally, the pellets were resuspended in phosphate-buffered saline (PBS) and stored at –80 °C for subsequent analysis.
Isolation of exosomes by means of markers CD63 and CD81
Exosomal markers CD63 and CD81 were detected by flow-cytometric analysis. Exosomes from serum or a cell culture medium were incubated with 5 µL of an antibody to CD81 (PE-Cy7 conjugate; eBioscience, San Diego, CA, 1:100) or to CD63 (FITC conjugate; eBioscience, San Diego, CA, 1:100) for 30 min at 4 °C away from light. Then, these exosomes were resuspended in the 400 μL of a buffer for flow staining. The percentage of exosomes was determined by flow cytometry, and the results were analyzed in the CXP software.
Transmission electron microscopic analysis
For transmission electron microscopy, the isolated exosomes were dissolved in a droplet of 4% paraformaldehyde in PBS, and a drop of the suspension was placed on a carbon-coated copper grid for 5 min, then removed from the grid, and we drained the excess liquid with filter paper. The grid was stained with saturated uranyl acetate for 5 min, allowed to dry for 10 min, and then examined under a transmission electron microscope (Hitachi H-7600; Hitachi Ltd., Tokyo, Japan) at 100 kV.
Particle size analysis
Exosome size distribution was tracked using the qNano system (Izon Science, Christchurch, New Zealand). Exosomes were diluted in PBS, and the size was detected by means of the NP100 nanopores and CPC100 calibration particles. Finally, the data were analyzed by means of the Izon Control Software Suite (version 2.2).
Quantitative reverse-transcription RT-PCR
Total RNA (2.5 µg) in the exosomes isolated from cell culture medium or serum was reverse-transcribed into cDNAs (Sangon Biotech, Shanghai) and subjected to quantitative PCR. The primer sequences for RT-PCR amplification were as follows: integrin β4, 5'-CTGGTCTTCTCCACCGAGTCA-3' (F), 5'-ATCGTTGCGGCTCATGATG-3' (R); GAPDH, 5'-TGGGTGTGAACCATGAGAAGT-3' (F), 5'-TGAGTCCTTCCACGATACCAA-3' (R). The expression level of GAPDH served as a control. The 2–ΔΔCt method was used for relative quantification of target genes. All the reactions were conducted in triplicate.
Western blotting
Exosomes isolated from a cell culture medium were homogenized and lysed in RIPA lysis buffer, and protein concentration was quantified by means of the BCA kit (Thermo Scientific). Next, the samples were resolved by SDS-PAGE on a 5% gel, and then transferred to a porous polyvinylidene fluoride membrane (Millipore, USA). The primary antibodies used in the present study were specific to the following proteins: integrin β4, phospho (p)-PI3K, PI3K, p-Akt, Akt, p-mTOR, mTOR, p16, p21, p27, and β-actin (Abcam, USA). The membranes were incubated with anti-mouse IgG antibodies (1:10,000) (secondary antibodies) with gentle shaking. Finally, each specific protein was visualized with a chemiluminescence detection system. Quantitative data were analyzed in the Quantity One software.
Cell proliferation assays
Cell proliferation was measured by the CCK-8 assay (Dojindo, Japan). After cell incubation, the CCK-8 reagent was added into each well for 1.5 h incubation. A microplate reader was used to measure the absorbance (OD value) at a wavelength of 450 nm. A colorimetric assay was performed, and all the experiments were conducted three times.
A knockdown of integrin β4 and siRNA transfection
Integrin β4 silencing was implemented by means of the following siRNA sequence: 5’-GTGGATGAGTTCCGGAATAAA (Hilden, Germany). Cell transfection was carried out using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). An siRNA scrambled sequence was included as a control (Santa Cruz Biotechnology). Western blotting and qRT-PCR were performed to confirm knockdown efficiency.
Statistical analysis
All the data are presented as the means ± standard deviation. Student’s t test or One-way ANOVA was employed to test the significance of differences between groups. Spearman’s rank correlation was calculated to assess the relation between a tumor stage and integrin β4 expression in the serum. Data with P<0.05 were considered statistically significant.
Results
Integrin β4 concentration in the serum from patients with osteosarcoma
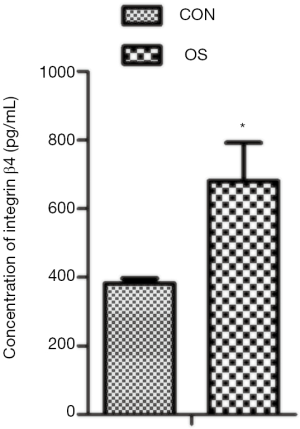
To explore the changes in integrin β4 concentration, we measured the level of integrin β4 in the serum from patients with osteosarcoma and healthy donors by the ELISA. Figure 1 shows that the concentration of integrin β4 in the serum of patients with osteosarcoma was higher than that in the healthy donors (Student’s t test; t=–17.95, P<0.01). We next analyzed the correlation between the grade of osteosarcomas and the concentration of integrin β4 and found that a high serum integrin β4 significantly correlated with an advanced tumor stage (Spearman’s rank correlation; r=0.673, P<0.01). Thus, it can be said that integrin β4 change may be involved in osteosarcoma progression.
Integrin β4 expression in the exosomes isolated from serum (Figure 2)
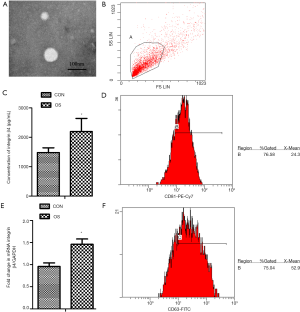
To further examine the effect integrin β4 on osteosarcoma progression, we isolated exosomes from the serum of patients with osteosarcoma and healthy donors. Transmission electron microscopy was employed to verify the isolation of the exosomes from the serum. The results revealed that images of round microvesicles with diameters of 50–100 nm were captured successfully (Figure 2A). We examined exosomal markers CD63 and CD81 by flow cytometry and confirmed that the exosomes expressed CD63 and CD81 at 76.58% and 75.04% prevalence, respectively (Figure 2B,D,F). ELISA and RT-PCR were carried out to evaluate the level of integrin β4 in the exosomes. The results showed that integrin β4 in the exosomes from patients with osteosarcoma was significantly upregulated as compared with that in the healthy donors (ELISA, Student’s t-test; t=–10.06, P<0.01; RT-PCR, Student’s t-test; t=–21.45, P<0.01; Figure 2C,E). These results suggested that integrin β4 in the exosomes participates in the osteosarcoma progression.
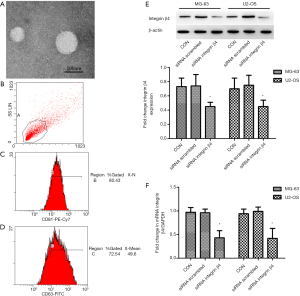
siRNA-silenced integrin β4 expression in the exosomes isolated from the cell culture medium
We silenced integrin β4 in U2OS and MG63 cells by means of a specific siRNA sequence and observed reduced integrin amounts in their exosomes and then measured the expression of integrin β4 by Western blotting and RT-PCR. As is shown in Figure 3A, the sizes of these exosomes were mostly ~70–100 nm. The positivity rates of exosomal markers CD63 and CD81 were 80.43% and 72.64%, respectively (Figure 3B,C,D). These results indicated that exosomes were successfully isolated by differential centrifugation from the two cell lines. The protein and mRNA levels of integrin β4 in the exosomes isolated from MG-63 cells [one-way ANOVA, Western blotting; F(2,21) =15.50, P<0.001; RT-PCR; F(2,21) =58.14, P<0.001] and U2-OS cells [one-way ANOVA, Western blotting; F(2,21) =12.15, P<0.001; RT-PCR; F(2,21) =38.13, P<0.001] were downregulated by siRNA as compared with the siRNA scrambled group (Figure 3E,F). These results suggested that the specific siRNA sequence effectively reduced the expression of integrin β4 in the exosomes isolated from the MG-63 and U2-OS cells.
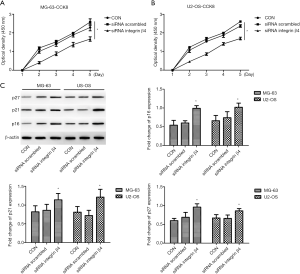
The knockdown of integrin β4 inhibits osteosarcoma cell proliferation in MG-63 and U2-OS cells
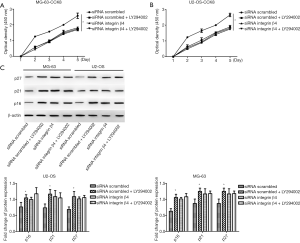
One study revealed that integrin β4 is involved in cancer cell proliferation (9), and then we selected these cell lines transfected with integrin β4 siRNA that causes a transient knockdown to perform CCK-8 cell assays. As illustrated in Figure 4A,B, the knockdown integrin β4 significantly inhibited proliferation of both MG-63 and U2-OS cell lines. Proteins p16, p21, and p27 play an important part in the progression of malignant tumors and can inhibit cell proliferation by binding to a variety of cyclins and cyclin-related protein kinases to hinder the procession of the cell cycle (15). Thus, we measured the protein expression of p16, p21, and p27 and found that siRNA significantly increased the p16, p21, and p27 protein levels in both MG-63 and U2-OS cell lines as compared with that in the siRNA scrambled group (P<0.001, P=0.006, P<0.001 for p16, p21, and p27 in MG-63 cells; P=0.001, P<0.001, P<0.001 for p16, p21, and p27 in U2OS cells, respectively; Figure 4C). Taken together, these results indicated that the knockdown of integrin β4 inhibited osteosarcoma cell proliferation both in MG-63 and U2-OS cells.
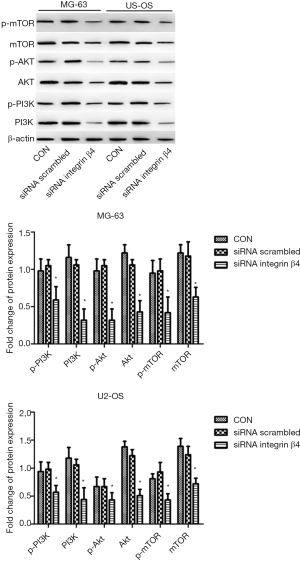
The effect of PI3K-Akt-mTOR signaling pathway on the osteosarcoma proliferation
One study gave a clue that the PI3K-Akt-mTOR signaling pathway crucially participates in the osteosarcoma proliferation (16). In the current study, we tested whether integrin β4 regulated osteosarcoma proliferation via PI3K-Akt-mTOR pathway signaling. Surprisingly, we found that after the knockdown of integrin β4, the protein levels of p-PI3K, PI3K, p-Akt, Akt, p-mTOR, and mTOR all remarkably decreased in both MG-63 and U2-OS cells as compared with the controls (all P<0.05; Figure 5). To confirm these results, we also used the PI3K inhibitor LY294002–treated MG-63 and U2-OS cells to examine the change in osteosarcoma cell proliferation. As presented in Figure 6A,B, the cell proliferation inhibition was totally reversed by the PI3K inhibitor LY294002 through decreasing the level of β4 integrin in MG-63 and U2-OS cell lines. These markers of proliferation—p16, p21, and p27—were also upregulated by LY294002 (all P<0.05), while there was no significant effect of LY294002 on the p16, p21 and p27 levels in the siRNA integrin β4 group (all P>0.05) (Figure 6C). In conclusion, these results suggested that LY294002 mimics the effect of integrin knockdown.
Discussion
Exosomal integrins have been reported to be deeply involved in the tumorigenesis and malignant progression of osteosarcoma (17,18). However, the underlying mechanism is poorly understood. In the present study, we sought to understand the involvement and molecular mechanisms of action of integrin β4 in the regulation of osteosarcoma cell proliferation. Our data suggested that the concentration of integrin β4 in the serum of patients with osteosarcoma was higher than that in the healthy donors, and the high integrin β4 level in the serum significantly correlated with advanced tumor stage. We next isolated exosomes from the serum of patients with osteosarcoma and healthy donors and found that integrin β4 in the exosomes from patients with osteosarcoma was also significantly more abundant as compared with that in the healthy donors. Exosomes are cell-derived small membrane microvesicles with a diameter of 40–150 nm (12)—present in the body fluids such as blood and urine—and contain a variety of biomolecules such as proteins, lipids, RNA, and DNA (13). Exosomes have been known to mediate tumor progression, such as activation of fibroblasts, promotion of angiogenesis, and enhancement of tumor cell proliferation and invasiveness as well as chemoresistance (10,17). Integrin β4 has been reported to be highly expressed in osteosarcoma cell lines and high-grade tumors from patients, at metastatic sites and in primary tumors (19). Furthermore, integrin β4 binds to multiple oncogenic receptor tyrosine kinases, such as EGFR, ErbB2/Neu, and Met, and promotes cell survival, proliferation, invasion, and migration (20-22). These data suggest that exosomal integrin β4 is involved in osteosarcoma progression.
To verify these data, MG-63 and U2-OS cells were applied to investigate the mechanism of action of integrin β4 in osteosarcoma. The results of Western blotting and RT-PCR demonstrated that β4 integrin was highly upregulated in both MG-63 and U2-OS cell lines. When we silenced integrin β4 by a specific siRNA sequence, in the exosomes isolated from the culture supernatants of both knockdown cell lines, the integrin β4 expression was lower as compared with the supernatant of the control cells. Furthermore, we measured the effect of the integrin β4 knockdown on osteosarcoma cell proliferation and found that this knockdown significantly inhibited the proliferation of both MG-63 and U2-OS cell lines. Accordingly, the protein expression of p16, p21, and p27, which are markers of cell proliferation and can inhibit cell proliferation, was also upregulated by siRNA targeting integrin β4 in both MG-63 and U2-OS cell lines. Previous works have reported that integrin β4 can activate PI3K-Akt (23), PKC (24), and Ras-ERK (25) signaling pathways, which are associated with cell migration and proliferation. Our present results indicate that siRNA to integrin β4 significantly downregulated the protein expression of p-PI3K, PI3K, p-Akt, Akt, p-mTOR, and mTOR in both MG-63 and U2-OS cell lines. To confirm these results, we also employed the PI3K inhibitor LY294002 to treat MG-63 and U2-OS cells and to examine the possible change of osteosarcoma proliferation. The data showed that the cell proliferation inhibition was totally reversed by LY294002 in both MG-63 and U2-OS cell lines. The markers of proliferation—p16, p21, and p27—were also upregulated by LY294002. In conclusion, these findings suggest thatβ4 integrin promotes cell proliferation probably via the PI3K-Akt-mTOR signaling pathway.
Conclusions
The present study for the first time demonstrated that β4 integrin is highly upregulated in osteosarcoma cell lines and high-grade osteosarcoma tumors from patients, and the larger amount of β4 integrin promotes cell proliferation (osteosarcoma progression) probably via the PI3K-Akt-mTOR signaling pathway.
Acknowledgments
Funding: This study was supported by the National Natural Science Foundation of China (81572699).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.09.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study protocol was approved by the ethics committee of the Xijing Hospital, The Fourth Military Medical University (approval No. ChiCTR-OPC-17010758). Written consent for participation in the study was obtained from all the participants or, where participants were children, from a parent or guardian.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Anderson ME. Update on survival in osteosarcoma. Orthop Clin North Am 2016;47:283. [Crossref] [PubMed]
- Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res 2009;152:3. [Crossref] [PubMed]
- Hu Y, Ylivinkka I, Chen P, et al. Netrin-4 promotes glioblastoma cell proliferation through integrin beta4 signaling. Neoplasia 2012;14:219-27. [Crossref] [PubMed]
- Kariya Y, Kariya Y, Gu J. Roles of Integrin alpha6beta4 Glycosylation in Cancer. Cancers (Basel) 2017;9:E79 [Crossref] [PubMed]
- de Pereda JM, Ortega E, Alonsogarcía N, et al. Advances and perspectives of the architecture of hemidesmosomes: Lessons from structural biology. Cell Adh Migr 2009;3:361-4. [Crossref] [PubMed]
- Kawakami K, Fujita Y, Kato T, et al. Integrin beta4 and vinculin contained in exosomes are potential markers for progression of prostate cancer associated with taxane-resistance. Int J Oncol 2015;47:384-90. [Crossref] [PubMed]
- Zhang X, Rozengurt E, Reed EF. HLA class I molecules partner with integrin beta4 to stimulate endothelial cell proliferation and migration. Sci Signal 2010;3:ra85. [Crossref] [PubMed]
- Chen Q, Xu R, Zeng C, et al. Down-regulation of Gli transcription factor leads to the inhibition of migration and invasion of ovarian cancer cells via integrin beta4-mediated FAK signaling. PLoS One 2014;9:e88386 [Crossref] [PubMed]
- Dydensborg AB, Teller IC, Groulx JF, et al. Integrin alpha6Bbeta4 inhibits colon cancer cell proliferation and c-Myc activity. BMC Cancer 2009;9:223. [Crossref] [PubMed]
- Soung YH, Thalia N, Cao H, et al. Emerging roles of exosomes in cancer invasion and metastasis. BMB Rep 2016;49:18-25. [Crossref] [PubMed]
- Kariya Y, Kariya Y, Gu J. Roles of laminin-332 and alpha6beta4 integrin in tumor progression. Mini Rev Med Chem 2009;9:1284-91. [Crossref] [PubMed]
- Denzer K, Kleijmeer MJ, Heijnen HF, et al. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci 2000;113:3365-74. [PubMed]
- Valadi H, Ekström K, Bossios A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007;9:654-9. [Crossref] [PubMed]
- Vlassov AV, Magdaleno S, Setterquist R, et al. Exosomes: Current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta 2012;1820:940-8. [Crossref] [PubMed]
- Yang Y, Guo JX, Shao ZQ, et al. Matrine inhibits bladder cancer cell growth and invasion in vitro through PI3K/AKT signaling pathway: An experimental study. Asian Pac J Trop Med 2017;10:515-9. [Crossref] [PubMed]
- Keremu A, Maimaiti X, Aimaiti A, et al. NRSN2 promotes osteosarcoma cell proliferation and growth through PI3K/Akt/MTOR and Wnt/beta-catenin signaling. Am J Cancer Res 2017;7:565-73. [PubMed]
- Qu JL, Qu XJ, Zhao MF, et al. Gastric cancer exosomes promote tumour cell proliferation through PI3K/Akt and MAPK/ERK activation. Dig Liver Dis 2009;41:875-80. [Crossref] [PubMed]
- Zhao F, Liu C, Hao YM, et al. Up-regulation of integrin α6β4 expression by mitogens involved in dairy cow mammary development. In Vitro Cell Dev Biol-Animal 2014;51:287-99.
- Wan X, Kim SY, Guenther LM, et al. Beta4 integrin promotes osteosarcoma metastasis and interacts with ezrin. Oncogene 2009;28:3401-11. [Crossref] [PubMed]
- Mariotti A, Kedeshian PA, Dans M, et al. EGF-R signaling through Fyn kinase disrupts the function of integrin alpha6beta4 at hemidesmosomes: role in epithelial cell migration and carcinoma invasion. J Cell Biol 2001;155:447-58. [Crossref] [PubMed]
- Guo W, Pylayeva Y, Pepe A, et al. β4 Integrin amplifies ErbB2 signaling to promote mammary tumorigenesis. Cell 2006;126:489-502. [Crossref] [PubMed]
- Trusolino L, Bertotti A, Comoglio PM. A signaling adapter function for alpha6beta4 integrin in the control of HGF-dependent invasive growth. Cell 2001;107:643-54. [Crossref] [PubMed]
- Shaw LM, Rabinovitz I, Wang HH, et al. Activation of phosphoinositide 3-OH kinase by the α6β4 integrin promotes carcinoma invasion. Cell 1997;91:949-60. [Crossref] [PubMed]
- Rabinovitz I, Alex T, Mercurio AM. Protein kinase C–dependent mobilization of the α6β4 integrin from hemidesmosomes and its association with actin-rich cell protrusions drive the chemotactic migration of carcinoma cells. J Cell Biol 1999;146:1147-60. [Crossref] [PubMed]
- Mainiero F, Murgia C, Wary KK, et al. The coupling of α6β4 integrin to Ras–MAP kinase pathways mediated by Shc controls keratinocyte proliferation. EMBO J 1997;16:2365-75. [Crossref] [PubMed]


