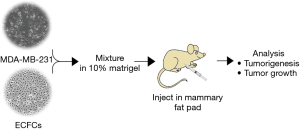
Exploring protocol for breast cancer xenograft model using endothelial colony-forming cells
Introduction
Breast cancer is the most common cancer in women worldwide. Approximately 15% to 25% of all breast cancer cases are classified as triple-negative breast cancer (TNBC), in which estrogen receptors, progesterone receptors and human epidermal growth factor receptor 2 (HER2) are not expressed (1). Due to the limited number of existing receptor and hormone targeting therapies for TNBC, it is considered an intractable type of cancer. In fact, the survival of patients with TNBC has not improved over the past two decades, and thus, intensive research is being undertaken to overcome the limitations of current therapies. However, the currently used experimental animal models are incapable of mimicking the tumor microenvironment, leading to poor understanding about tumor progression. Thus, a suitable animal model is needed to accurately predict clinical outcome.
The proliferation of tumor-initiating cells is characterized by abnormal cell division and angiogenesis. Thus, the formation of new blood vessels is of considerable importance in the context of tumor proliferation and growth. Endothelial progenitor cells (EPCs) are not only involved in fetal angiogenesis but are also known to participate in angiogenesis in backgrounds of ischemia, cardiovascular disorder, cancer and other diseases (2). Endothelial colony-forming cells (ECFCs) classified as a population of the EPCs exhibit cobblestone morphology and are obtained from human peripheral blood mononuclear cells (hPBMNCs) or umbilical cord blood-derived circulating mononuclear cells (MNCs) cultured in collagen I-coated dishes (3). ECFCs demonstrate high proliferative capacity, telomerase activity, and in vivo vessel forming ability which are associated with the self-secretion of factors that promote vascular regeneration and repair. In addition, ECFCs differentiate into endothelial cells.
TNBC is an especially aggressive cancer and metastasizes to lung, brain and other organs (4,5). For this reason, transplanted MDA-MB-231 cells are often used in xenograft models for preclinical testing (6,7). However, they cannot accurately mimic tumor microenvironments. In the present study, we established a TNBC xenograft model in mice based on the implantation of MDA-MB-231 and ECFCs and assessed the effects of ECFCs on MDA-MB-231 cell-derived tumor progression.
Methods
Cell culture
MDA-MB-231 cells (human breast cancer cell-line) were incubated in RPMI-1640 (GenDEPOT, Barker, TX) containing 10% fetal bovine serum (Young In Frontier, Seoul, Korea) and 1% penicillin-streptomycin (GenDEPOT) in a 5% CO2 incubator at 37 °C. Human ECFCs were isolated from human peripheral blood obtained from a biobank after securing Institutional Review Board approval of the study protocol. MNC fractions were separated from whole blood samples and ECFCs were isolated from MNCs using CD31-coated magnetic beads (Invitrogen, CA, USA) as previously described by Melero-Martin and Bischoff (8). Isolated ECFCs (Figure S1) were expanded on 1% gelatin-coated plates (BD Biosciences, CA, USA) using EGM-2 in the absence of hydrocortisone (Lonza, Basel, Switzerland), but supplemented with 10% FBS (Atlas Biologicals, CO, USA) and 1% glutamine-penicillin-streptomycin (Gibco, MA, USA). ECFCs between passages 7 and 10 were used for all experiments.
Preparation of animals
Balb/c-nude mice (female, 5-week-old) were purchased from SLC (Shizuoka, Japan). All animal procedures were approved beforehand by the Institutional Animal Care and Use Committee of Duksung Women’s University in accordance with guidelines for the care and use of laboratory animals issued by the university. Briefly, the experimental animal cages were maintained at 22±4 °C and approximately 50% to 60% relative humidity under a 12-h light/dark lighting cycle. Laboratory diet and drinking water were provided ad libitum. Mice were used after a week of acclimatization.
Establishment of xenograft models
Protocol 1
Balb/c nude mice were randomly divided into two groups, a control (CON 1) and co-transplantation (Co-treat 1) (each group, n=4). MDA-MB-231 cells and ECFCs were harvested and counted using a hemocytometer (Marienfeld, Germany). In the control group, MDA-MB-231 cells (5×107 cells/mL) in 10% matrigel (Corning, NY, USA) were prepared and placed on ice and in the co-transplantation group, a mixture of MDA-MB-231 cells (5×107 cells/mL) and ECFCs (1×107 cells/mL) in 10% matrigel were prepared and placed on ice. Then, MDA-MB-231 cells (100 L, 5×106 cells) with or without ECFCs (1×106 cells) were orthotopically transplanted into a mammary fat pad in each mouse.
Protocol 2
Protocol 2 was identical to protocol 1 except for the number of MDA-MB-231 cells that were injected into the mice. Mice were divided into the same two groups as in protocol 1 (control group, n=11; co-transplantation group, n=25). The number of injected ECFCs was identical as in protocol 1 but MDA-MB-231 cells were reduced to 3×105 cells/mL. As described in protocol 1, 100 L of prepared cells (3×104 MDA-MB-231 cells with or without 1×106 ECFCs) were orthotopically injected into a mammary fat pad in each mouse.
Evaluation of xenograft models
The evaluated parameters were tumorigenicity, tumor growth and tumor weight. Tumorigenicity was assessed by visual examination. Tumor volumes were determined by recording a measurement using a caliper three times per week for 5 weeks. Volumes were calculated using an equation [tumor volume (mm3) = (tumor length) × (tumor width)2 × 0.5] (9).
An image of each mouse was acquired before sacrifice and tumor tissues were isolated and weighed.
In vivo staining by tail vein injection of ulex europaeus agglutinin-I (UEA-I)
UEA-I is a lectin-specific for human endothelium. Rhodamine-conjugated UEA-I (Vector laboratories, CA, USA) was suspended in a saline solution supplemented with 1 mM CaCl2. UEA-I (50 g/100 L/mouse) was injected intravenously and allowed to circulate for 10 min before harvesting tumor tissues. Mice were euthanized and tumor tissues were harvested, fixed in 10% buffered formalin overnight, placed in 30% sucrose for an additional overnight incubation, embedded in OCT, frozen, and cryosectioned (12 µm-thick sections). The frozen sections were mounted with Vectashield containing DAPI (Vector Laboratories, CA, USA). Perfused human vessels were identified as UEA-I-labeled lumenal structures (red) by confocal laser scanning microscope (Carl Zeiss, Oberkochen, Germany). A 40×/1.25 oil objective was used (scale bar =50 µm).
Western blot analysis
Tumor tissues harvested from mice were homogenized and lysed in RIPA buffer (GenDEPOT, Barker, TX, USA) containing protease inhibitors (Xpert protease inhibitor cocktail solution, GenDEPOT), and phosphatase inhibitors (Roche, Germany). Tissue lysates were boiled in 5× sample buffer and separated by 10% SDS-PAGE. Proteins were transferred onto a PVDF membrane (Merck, Germany) using a semi-dry electroblotter (Peqlab, Germany). Membranes were blocked with 5% skim milk in TBST (50 mM Tris-HCl pH7.4, 150 mM NaCl, 0.1% Tween 20) for 1 h, and incubated overnight with primary antibodies at 4 °C. After washing with TBST, the membrane was re-incubated with a secondary antibody for 2 h at room temperature. After washing the membrane, immunoreactive proteins were visualized using ECL reagents that were detected with a ChemiDoc Imaging System. The antibodies and the ratios at which they were used were MMP-2 (1:1,000, Millipore), VEGF (1:1,000, Abcam), β-actin (1:5,000, Sigma), anti-mouse IgG (H+L) horseradish peroxidase conjugate (1:3,000, Bio-Rad) and anti-rabbit IgG (H+L) horseradish peroxidase conjugate (1:3,000, Bio-Rad).
Immunohistochemical assay
Tissues harvested from mice were immediately molded by the OCT compound and sliced to a thickness of 5 µm on a cryostat. Frozen sections on glass slides were washed with tap water and incubated in 3% H2O2 in methanol. After washing with PBS, tissue sections were blocked with 10% BSA in PBST for 1 h at room temperature and were incubated with anti-Ki-67 primary antibody (Abcam, 1:500) at 4 °C overnight. After washing with PBS, sections were conjugated with anti-mouse secondary antibody (Bio-Rad, 1:100) for 2 h. The sections were washed and incubated with another antibody (ABC kit, Vector Laboratories, Inc., CA). After washing with TBS, sliced tissue sections were stained with DAB (Vector Laboratories, Inc.). Stained sections were detected using a microscope (Leica, Germany).
Statistics analysis
Differences between the two groups were regarded as significant when P<0.05 by the Student’s t-test.
Results
Co-transplantation of MDA-MB-231 cells and ECFCs was investigated to better mimic the tumor environment. A schematic of the process used is provided in Figure 1. The two protocols were compared with respect to several parameters for optimization. In particular, we evaluated the effects of ECFCs on MDA-MB-231-derived tumor progression in vivo.
Protocol 1
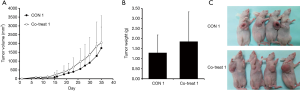
To investigate MDA-MB-231-derived tumor progression in the presence or absence of ECFCs, 5×106 MDA-MB-231 cells were injected, as described previously (10-13). Tumorigenesis was assessed by visual inspection. Tumors developed in all animals of the CON 1 and Co-treat 1 groups and the durations to reach a tumor volume of 100 mm3 were 15.63±2.87 and 12.86±7.74 days, respectively, but this difference was not significant (Figure 2A). Furthermore, tumor weights were also not significantly different between the two groups (Figure 2B) and no intergroup difference was evident upon visual observation (Figure 2C). These results suggested that ECFCs did not promote MDA-MB-231-derived tumor progression because 5×106 MDA-MB-231 cells were enough to establish the xenograft model without other factors as shown in previous studies (10-13).
Protocol 2
From the result of protocol 1, we hypothesized that the effects of ECFCs may be masked and a reduction in the number of MDA-MB-231 cells without affecting the number of ECFCs, may affect tumor progression. Therefore, MDA-MB-231 cells were injected at 3.0×105 cells/mouse, which is the minimum for developing a xenograft model (14). To determine effects on tumorigenesis, the number of mice in the CON 2 and Co-treat 2 groups was increased to 11 and 25, respectively, because the number of inoculated MDA-MB-231 cells was comparatively small. As shown in Figure 3A, incidence rates in CON 2 and Co-treat 2 groups were 63.6% and 72%, respectively. The odds ratio of tumorigenicity was 1.47. This result indicated that ECFCs enhanced the tumorigenicity of MDA-MB-231 cells in Balb/c nude mice. Furthermore, the duration to reach a tumor volume of 100 mm3 in the CON 2 and Co-treat 2 groups were 19.55±3.43 days and 13.98±2.86 days, respectively. Thus, tumor growth in the Co-treat 2 group was significantly higher than that in the CON 2 group (Figure 3B,C). These results indicate that ECFCs promoted tumor progression in our xenograft model.

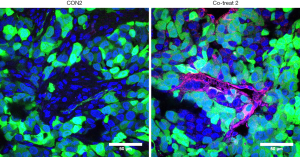
Perfused human blood vessels in tumor tissues co-injected with ECFCs using protocol 2
ECFC-mediated perfusion of human blood vessels was confirmed by in vivo staining through tail vein injection of UEA-I. As shown in Figure 4, perfused human blood vessels were detected within tumor tissues that were induced by co-injection of MDA-MB-231 cells and ECFCs (red in Figure 4, right panel). Whereas, no human vessels were detected in the tumor tissues that were induced by MDA-MB-231 cells alone (Figure 4, left panel). The green signal in both tissues indicated non-specific auto-fluorescence generated by MBA-MD-231 cells and adjacent host fibroblasts. This result suggests that ECFC-mediated human blood vessels may contribute toward supplying oxygen and nutrition to the cancer cells, which can enhance cell survival and proliferation, resulting in the significant increase in tumor growth compared with the cancer cell alone.
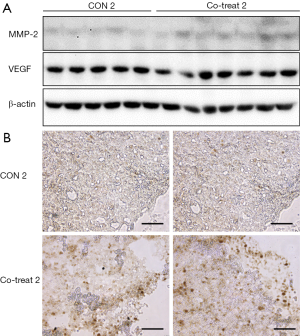
The effect of ECFCs on tumor progression in protocol 2
In protocol 2, co-transplantation of ECFCs with MDA-MB-231 cells showed a higher incidence rate and more rapid growth of tumors than with only MDA-MB-231 cells. For evaluation of tumor progression, we observed VEGF, MMP-2, and Ki-67 expression in tumor tissues. As shown in Figure 5A, VEGFR and MMP-2 expression slightly higher in the Co-treat 2 group than in the CON 2 group. Furthermore, Ki-67, which serves as a proliferation marker, was significantly overexpressed in the Co-treat 2 group. These results indicate that ECFCs enhanced the tumor progression of MDA-MB-231 cells.
Discussion
The MDA-MB-231 cell line is commonly used in in vitro and in vivo studies of TNBC. MDA-MB-231 cell-derived breast cancer xenograft models are easily generated without supplements, such as matrigel or estrogen pellets. MDA-MB-231 cells are an invasive, ductal carcinoma cell line that are prone to metastasis (15) and exhibit rapid growth upon transplantation. We hypothesized that the excessive number of MDA-MB-231 cells might be the reason for not observing tumor progression by ECFCs in protocol 1 (Figure 1). On the other hand, protocol 2, which used a smaller number of MDA-MB-231 cells, exhibited a lower tumor development rate and slower initial tumor growth in only the MDA-MB-231-derived xenograft model. However, ECFCs increased the tumor development rate and significantly promoted tumor growth in protocol 2. These results suggest that cell number is an important factor in tumor progression. In other words, when we artificially establish the xenograft model, a smaller number of MDA-MB-231 cells lead to tumorigenicity, with ECFCs supporting the TNBC xenograft model. We could consider the ratio of MDA-MB-231 and ECFCs as well as the cell number for the relationship between MDA-MB-231 and ECFCs. Nevertheless, we take priority of the cell numbers of MDA-MB-231 and ECFCs in this study. Based on several previous reports for reproducibility, we combined MDA-MB-231 and ECFCs. Several studies suggested that over one million MDA-MB-231 cells are required to develop a xenograft model for TNBC (10-13). Furthermore, over 100,000 ECFCs were also used for a vasculogenesis model (16,17). In protocol 2, unlike protocol 1, the differences in tumorigenicity and tumor growth were significant. The xenograft model with ECFCs significantly promoted tumor growth more than that without ECFCs (Figure 3). Unfortunately, the effect of ECFCs on the invasion of MDA-MB-231 cells was difficult to demonstrate in this model. However, we observed enhanced tumor proliferation, indicated by increased expression of Ki-67 in tumor tissues co-transplanted with MDA-MB-231 and ECFCs (Figure 5B). These observations suggest that the co-transplantation of MDA-MB-231 and ECFCs enhanced tumor progression in the xenograft models, and that protocol 2 mimicked the tumor microenvironment with more efficiency.
Conclusions
In the present study, a protocol for the establishment of an MDA-MB-231 cell xenograft model was optimized to evaluate the effect of ECFCs on breast cancer cell proliferation. Transplantation of 5×106 MDA-MB-231 breast cancer cells (protocol 1) resulted in achieving 100% tumor progression rate, regardless of the presence of ECFCs, and ECFCs exhibited only marginal effects on the xenograft model. On the other hand, ECFCs promoted tumor development and early growth in the mice that were injected with only 3×105 MDA-MB-231 cells (protocol 2). These results suggest that protocol 2 should be considered for evaluations of tumor progression and chemotherapeutic efficacy.

Acknowledgments
Funding: This work was supported by Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2016R1A6A1A03007648).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.09.09). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was waived. The experimental protocols were approved by the Institutional Research Ethics Committee (No. 2017-002-001) and the Institutional Animal Care and Use Committee (No. 2018-003-006) of Duksung Women’s University.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Anders C, Carey LA. Understanding and treating triple-negative breast cancer. Oncology (Williston Park) 2008;22:1233-9; discussion 1239-40, 1243.
- George AL, Bangalore-Prakash P, Rajoria S, et al. Endothelial progenitor cell biology in disease and tissue regeneration. J Hematol Oncol 2011;4:24. [Crossref] [PubMed]
- Critser PJ, Yoder MC. Endothelial colony-forming cell role in neoangiogenesis and tissue repair. Curr Opin Organ Transplant 2010;15:68-72. [Crossref] [PubMed]
- Criscitiello C, Azim HA Jr, Schouten PC, et al. Understanding the biology of triple-negative breast cancer. Ann Oncol 2012;23:vi13-8. [Crossref] [PubMed]
- Aysola K, Desai A, Welch C, et al. Triple Negative Breast Cancer - An Overview. Hereditary Genet 2013;2013:001 [PubMed]
- Chavez KJ, Garimella SV, Lipkowitz S. Triple negative breast cancer cell lines: one tool in the search for better treatment of triple negative breast cancer. Breast Dis 2010;32:35-48. [Crossref] [PubMed]
- Minn AJ, Gupta GP, Siegel PM, et al. Genes that mediate breast cancer metastasis to lung. Nature 2005;436:518-24. [Crossref] [PubMed]
- Melero-Martin JM, De Obaldia ME, Kang SY, et al. Engineering robust and functional vascular networks in vivo with human adult and cord blood-derived progenitor cells. Circ Res 2008;103:194-202. [Crossref] [PubMed]
- Kim KM, Lim HK, Shim SH, et al. Improved chemotherapeutic efficacy of injectable chrysin encapsulated by copolymer nanoparticles. Int J Nanomedicine 2017;12:1917-25. [Crossref] [PubMed]
- Kim ES, Kim SY, Koh M, et al. C-reactive protein binds to integrin α2 and Fcγ receptor I, leading to breast cell adhesion and breast cancer progression. Oncogene 2018;37:28-38. [Crossref] [PubMed]
- Kim ES, Cha Y, Ham M, et al. Inflammatory lipid sphingosine-1-phosphate upregulates C-reactive protein via C/EBPβ and potentiates breast cancer progression. Oncogene 2014;33:3583-93. [Crossref] [PubMed]
- Kashyap T, Argueta C, Unger T, et al. Selinexor reduces the expression of DNA damage repair proteins and sensitizes cancer cells to DNA damaging agents. Oncotarget 2018;9:30773-86. [Crossref] [PubMed]
- Li J, Gong X, Jiang R, et al. Fisetin Inhibited Growth and Metastasis of Triple-Negative Breast Cancer by Reversing Epithelial-to-Mesenchymal Transition via PTEN/Akt/GSK3β Signal Pathway. Front Pharmacol 2018;9:772. [Crossref] [PubMed]
- Lee E, Pandey NB, Popel AS. Lymphatic endothelial cells support tumor growth in breast cancer. Sci Rep 2014;4:5853. [Crossref] [PubMed]
- Cailleau R, Young R, Olivé M, et al. Breast tumor cell lines from pleural effusions. J Natl Cancer Inst 1974;53:661-74. [Crossref] [PubMed]
- Mena HA, Zubiry PR, Dizier B, et al. Acidic preconditioning of endothelial colony-forming cells (ECFC) promote vasculogenesis under proinflammatory and high glucose conditions in vitro and in vivo. Stem Cell Res Ther 2018;9:120. [Crossref] [PubMed]
- Kang KT, Lin RZ, Kuppermann D, et al. Endothelial colony forming cells and mesenchymal progenitor cells form blood vessels and increase blood flow in ischemic muscle. Sci Rep 2017;7:770. [Crossref] [PubMed]