Primary malignant melanoma of the esophagus: a population-based study
Introduction
Malignant melanoma most commonly occurs in dermatological lesions and primary malignant melanoma of the esophagus (PMME) is extremely rare. The incidence of PMME is approximately 0.2–0.3% of all esophageal carcinomas and 0.5% of malignant melanomas (1). PMME may originate from the melanocytes that exist in the esophageal squamous epithelium or basement membrane (2). PMME was first reported by Baur et al. in 1906 (3), and only a few cases from Asian populations have been recorded since then (4-7). Most PMME patients present with nonspecific symptoms similar to those of other primary esophageal tumors, including dysphagia, retrosternal pain and weight loss. The prognosis of PMME is dismal because of its high metastatic and recurrent potential (8). There are no standard treatment strategies or guidelines for PMME; however, surgical resection might be an effective treatment procedure, and the role of adjuvant therapy remains unclear (9). Due to the lack of data for western patients with PMME, its prognosis and characteristics have yet to be determined. Moreover, demographic feature and survival outcome associations between PMME and other histological subtypes of esophageal neoplasms have not been characterized until now. The Surveillance, Epidemiology, and End Results (SEER) database of the National Cancer Institute is the largest open-access database that provides reliable and detailed information on cancer statistics (10). In this study, we aimed to investigate the clinical characteristics and prognosis of PMME using the SEER database from 1973 to 2014. We further verified the classification system of esophageal epidemiology by conducting a comparison of the survival outcome of PMME with that of other subtypes of esophageal neoplasms.
Methods
Patient collection
This study utilized the SEER-18 registry databases, which currently covers approximately 30% of the United States population. SEER routinely collects the demographic, tumor site, stage at diagnosis, first course of treatment, and follow-up of vital status data. We retrieved data from 1973 to 2014 using SEER 8.3.5 software, and searched for all cases of PMME patients using the ICD-O-3 codes 8720-8799. In addition, the patients who were diagnosed with other subtypes of esophageal neoplasms during the same period were also identified.
Statistical analysis
Our analysis included age at diagnosis, sex, primary site, race, SEER summary stage, treatment to the primary site, months of survival and vital status. We employed Student’s t-test to analyze continuous data, and categorical variables were evaluated using the Chi-square test or Fisher’s exact test. A log-rank test was conducted to compare the Kaplan-Meier survival curves. Overall survival (OS) was measured from the date of the initial treatment to the date of death or the last day of follow-up. Cancer-specific survival (CSS) was defined as the time from diagnosis to death due to PMME. Univariate analyses were conducted on all of the variables included in the study. Variables that yielded P<0.5 in the univariate analysis were used for the multivariate analyses. Multivariate analyses with the Cox proportional hazards model were performed to evaluate the covariate effect on OS and CSS. Hazard ratios with 95% confidence intervals were employed to quantify the strength of the association between the predictors and survival. A two-tailed P value of <0.05 was considered statistically significant. All statistical calculations were performed using SPSS 22.0 (IBM, NY, United States), and images of the statistical results were produced using GraphPad Prism 7.0 (GraphPad Software, San Diego, CA, USA).
Results
Patient characteristics
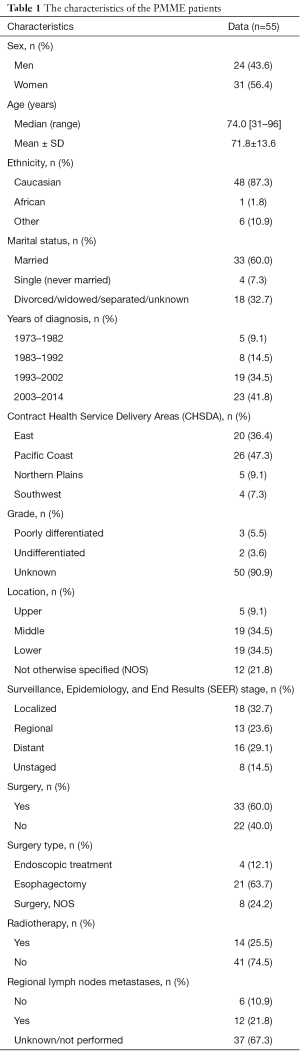
We identified a total of 83,448 esophagus cancer cases in SEER between 1973 and 2014. Among them, 55 PMME patients were included, and their clinicopathological characteristics are shown in Table 1. The mean age at diagnosis was 71.8±13.6, and there was a female prevalence (1.3:1). Caucasian patients were the predominant ethnicity (48, 87.3%), and only one African case was identified. Another 6 patients of American Indian, Asian and Pacific Islander races were included. Most of these patients were from the Eastern and Pacific Coast region (46, 83.7%), and were married at diagnosis (33, 60.0%). In terms of the primary site, the tumors were located mainly in the middle to lower thoracic esophagus (38, 69.0%). Because there is no unified staging system for PMME, we used the SEER staging system for our analyses. The tumors were classified as localized (PMME infiltrating through the basement membrane of the epithelium but not spreading beyond the boundaries of the esophagus), regional (PMME extending beyond the wall of the esophagus or the presence of regional lymph node metastases) or distant (the presence of a metastatic tumor anywhere beyond what we defined as regional disease). Among the 55 included patients, 18 patients were categorized with localized stage, 13 had regional stage, whereas 16 had distant stage.
Full table
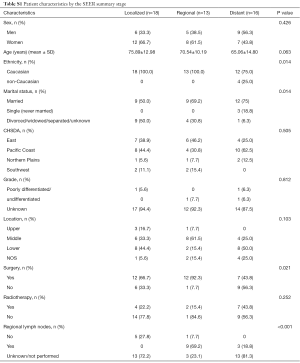
Table S1 shows the characteristics of PMME across the SEER staging system. Patients with early stage PMME (localized or regional) were more likely to undergo surgical resection and were less likely to have lymph nodes metastases. Caucasian patients had a greater probability of suffering from PMME. However, neither sex nor age was significantly different among PMME patients with different stage.
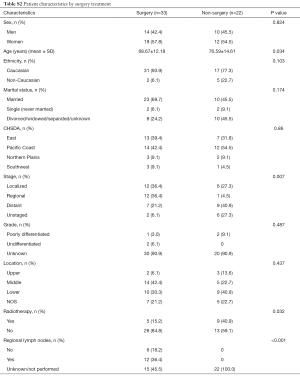
Regarding the treatment, cancer-directed surgery was performed for 33 (60.0%) cases, while 14 (25.5%) cases received radiation therapy. Esophagectomy (21, 63.7%) was the primary type of surgery for those who received surgical resection; 4 (12.1%) received endoscopic treatment, and the resection information for 8 (24.2%) patients was incomplete. We investigated the characteristics pf the patients according to their surgical information. The comparison analysis showed that younger patients with early stage were more likely to receive surgery. There was no significant difference in the location of the primary site between patients who underwent surgical resection or not (see Table S2).
Patient survival
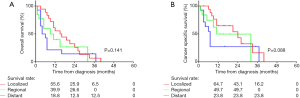
The median OS was 9 months for patients with PMME, and the 1- and 3-year survival rates were 36.4% and 7.3%. Compared with other esophageal histological types, the OS of PMME was extremely inferior (see Figure 1A). Unfortunately, no case with a survival of more than 5 years was found. The median CSS of PMME was 11 months, and the 1- and 3-year survival rates were 43.0% and 17.1%, respectively. However, the CSS was not significantly different between patients with PMME and other histological types (median CSS: 11 vs. 13 months, P=0.168) (Figure 1B). We further estimated the differences in the survival outcomes among patients with PMME, squamous cell carcinoma (SQ), adenocarcinoma (AD) and small cell carcinoma (SCC). We found that the overall survival of PMME patients resembled that of SQ patients (HR =0.798; 95% CI, 0.603–1.057; P=0.116) but inferior than that of AD patients (HR =0.565; 95% CI, 0.426–0.747; P<0.001). The comparison of CSS between these histological subtypes also revealed the same results (Figure 2A,B). Of note, only 47 of 55 PMME cases with complete survival data were included in our study. Kaplan-Meier curves for survival stratified by SEER summary stage classification are shown in Figure 3A,B. Neither OS nor CSS differed significantly among different stages of PMME.


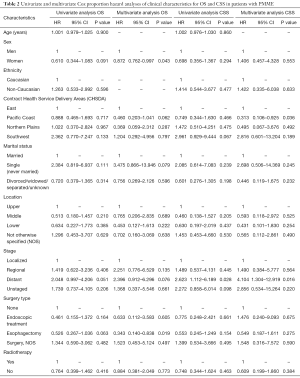
We analyzed variables potentially influencing OS using univariate Cox proportional hazards analyses and found that the sex, region, marital status, primary site of tumor, SEER summary stage, surgery type and radiation therapy of the patients were significant prognostic factors. Multivariate Cox analyses showed that women had better survival outcomes than men (HR =0.872; 95% CI, 0.762–0.997; P=0.043). However, no significant difference was noted among patients with different stages. Similar results were found for the location of the primary tumor site. In terms of treatment, patients who received radiotherapy had shorter survival times, but no significant difference was found (HR =0.884; 95% CI, 0.381–2.049; P=0.773). Conversely, radical esophagectomy was an independent prognostic factor for PMME (HR =0.343; 95% CI, 0.140–0.838; P=0.019). In the multivariate analysis for CSS, there were no prognostic factors with significant differences among PMME patients (Table 2).
Full table
Discussion
PMME is certainly a rare disease, with no more than 370 cases reported through 2014 in the world (11). The majority of cases have been reported in Asian patients, especially the Japanese population. Hence, our understanding of the clinical characteristics and prognosis of Western PMME patients remains limited. In the present study, we described the clinicopathological features and outcomes of this population by extracting data for 55 patients from the SEER database between 1973 and 2014. However, we noted that the earliest case was identified in 1978. We also found that the incidence of PMME increased throughout the study period, as shown in Table 1, which might have contributed to the development of diagnostic approaches and awareness of this specific malignancy in clinical practice.
In our study, Caucasian patients were predominant, which is consistent with the ethnicity distribution of the Western population. We found that patients over 60 years-of-age accounted for the largest proportion of PMME patients. The median age at diagnosis was 74 and ranged from 31 to 96. The mean age of all patients was 71.8 years. Previous studies suggested that PMME often affects patients between their sixth and seventh decades of life, which was younger than the age of the Western patients in our research (1,2). A total of 31 female patients and 24 male patients were included in this study, and the female/male ratio was 1.3:1. However, previous reports showed that PMME is prevalent among males, with a ratio of 2–3:1, which was in contrast to our study. To investigate this difference, we searched published case series written in English with more than 5 patients from the PubMed database and the results are shown in Table 3. Lohmann et al. reported a similar sex ratio (5:5), which was also suggested by Sanchez and his colleagues (12,13) (2:3). Previous literature suggests that male patients account for a larger proportion of Asian PMME cases (4,7,8,14), while our study revealed that female patients were more likely to suffer from PMME in the Western population. Furthermore, another explanation of this difference might be the relatively small sample sizes. Referring to the regions, the majority of the patients came from the Eastern and Pacific Coast region, which indicates that the patients in this region received developed health care and were more likely to be able to afford the medical expenses.

Full table
Because that there is no standardized AJCC classification for PMME, we employed the SEER summary stage to categorize the included cases. One of the advantages of utilizing the SEER summary stage is the assurance of a consistent staging definition over time. We found that patients with advanced stage PMME (regional and distant) accounted for a large proportion of all patients (29, 61.7%). Iwanuma et al. reviewed the clinicopathological features and biological behavior of PMME and reported that 40% to 80% of cases had local regional lymph node metastases at the time of diagnosis (2). Then, Makuuchi’s group confirmed that PMME has a high metastatic potential. Based on an analysis of 134 cases collected throughout Japan from 1998–2007, the authors found that the incidence of lymph node metastasis at the initial diagnosis is approximately 65% (1). Along with these reports, we suggest that PMME patients are usually diagnosed at a late stage, and the early detection of this aggressive malignancy should be considered. Regarding the primary sites, we found that the middle and lower thoracic esophagus are predominantly affected. This finding also agrees with previous studies indicating, that more than 90% of PMME lesions are located in the distal 2/3 of the esophagus (2,11,13). Endoscopy discovery of these lesions may support the diagnosis of PMME, but a pathological analysis and immunohistochemical examination are necessary for a definitive diagnosis.
The prognosis of PMME patients is extremely poor. The median OS was 9 months, and the 1- and 3-year OS rates were 36.4% and 7.3%. The median CSS was 11 months, and the 1- and 3-year CSS rates were 43.0% and 17.1%, respectively. According to previous reports, although the 5-year OS has increased from 4.2% in 1990 to 37% in the new century, PMME is still considered a clinically fatal disease (Table 3). Bisceglia and his colleagues analyzed the survival outcome of 99 PMME patients who were diagnosed in the 10-year period and found that the prognosis was dismal at only 4.5%. There are no more than 20 recorded cases with a survival of more than 5 years in the literature through 2015 (1). Unfortunately, no patient living longer than 5 years was identified in the present study, which indicates that the survival of Western patients might be worse than that of their Asian counterparts. Moreover, we found that PMME had a worse prognosis than other esophageal cancers. The survival of PMME was significantly inferior to that of AD patients. Consistent with the published literature (2) that suggests the clinicopathological features of PMME resemble those of SQ of the esophagus, we found no significant differences in survival between these types. However, we noted that the sample size of PMME patients was much smaller than that of non-PMME patients; the histological type and survival association should be conducted in a larger cohort of PMME patients in the future.
Sex was an independent prognostic factor for patients with PMME in both univariate and multivariate Cox analyses of OS. Compared with men, women had significantly better outcomes. Bohanes et al. used a large population database to investigate the influence of hormonal status on survival in patients with esophageal cancer and demonstrated that sex is an independent prognostic marker for patients with either metastatic or locoregional esophageal neoplasms (15). Then, an Asian population-based study confirmed that the prognosis of males with esophageal cancer is significantly worse than that of females after esophagectomy (16). Likely the survival benefit of sex is due to multiple clinical factors, such as the different health-care seeking behaviors of men and women. According to a previous study (17), men might endure symptoms longer than women before seeking for medical care; therefore, they are more likely to be diagnosed with a higher tumor burden and worse prognosis. However, the results showed that ethnicity, age at diagnosis, location of the tumor, and marital status were not correlated with the OS of PMME. Moreover, the SEER summary stage was not an independent prognostic factor; one possible explanation might be that stage information was not available for 8 (14.5%) patients, and only 47 of 55 patients were included in the final analysis. We believe that the survival of early stage PMME patients would be better than that of advanced stage PMME patients, if more cases were included.
Due to the low prevalence of PMME, there is a lack of consensus on the treatment strategy for this malignancy. Surgical resection remains the primary option for resectable PMME patients. In our study, the majority of patients with localized and regional stage PMME received surgery. Esophagectomy, which is regarded as a radical resection type and could maximize the locoregional control, was performed in 63.7% of patients who underwent surgery. In the univariate analysis of OS, patients who underwent esophagectomy had a significantly better prognosis than those who did not undergo surgery. A multivariate Cox analysis also suggested that esophagectomy was an independent prognostic factor for PMME patients, aside from age, race, region, marital status and primary tumor site. The resection rate data from previous studies are shown in Table 3; we found that the R0 ratio improved from 30% in 1985 to nearly 80% in 2015. However, the 5-year survival rates of PMME after surgery remains dismal, with rates of approximately 20–40% reported in published studies (1,6). Yamamoto et al. indicated that the presence of recurrent disease and lymph node metastasis, even after curative resection, might result in the adverse outcome of PMME (11). In our study, only a few patients received radiotherapy as their adjuvant treatment, and radiation therapy was not associated with improved survival for OS or CSS. The median OS for those who underwent radiotherapy was 5 months. It has been well-accepted that malignant melanoma is relatively radio-resistant, and radiotherapy is generally considered as a palliative option for patients with unresectable disease. However, hypofractionated radiotherapy has been advocated as a possible treatment strategy for malignant melanoma in recent years (18); thus, we cannot completely exclude the possibility that radiotherapy may improve the local control of tumors for PMME patients. Another adjuvant therapy option for PMME patients is chemotherapy, such as dacarbazine-containing regimens. However, due to the lack of information about chemotherapy in the SEER database, we could not investigate the effect of chemotherapy on survival among PMME cases. Recently, novel methods, including molecular targeted therapy and peptide vaccination, have been conducted in some clinical trials for PMME patients and showed some effects (1). Despite the fact that interferon alpha is the only therapy approved for the adjuvant therapy of melanoma, few authors have reported PMME cases with favorable responses to the administration of anti-PD-1 (nivolumab, pembrolizumab) or anti-CTLA-4 antibodies (ipilimumab) (18). Moreover, the combination of radiotherapy and immunotherapy has a demonstrated substantial clinical benefit in patients with metastatic melanoma (19). This combination may also be effective for PMME patients. Moreover, according to the genetic alterations in oncogenes, melanoma patients can be classified and treated with immunotherapy. A recently published multicenter analysis suggested that although the mutation status of neuroblastoma rat sarcoma viral oncogene homologue (NRAS) showed no impact on the response rates to checkpoint inhibitor therapy among melanoma patients, the survival outcomes was significantly inferior for those with NRAS mutant than their wildtype competitors (20). The authors further suggested that additional MEK inhibition might improve the survival in patients with NRAS mutant melanoma. Considering the extremely rare occurrence of PMME, it would be difficult to verify the value of these treatments. Thus, the establishment of clinical guidelines and standard treatment strategies for PMME is far from being complete.
In this population-based study, we compared the clinical and histological features of PMME to other esophageal neoplasms. Recently, there are some publications focus on the comparison of prognosis between PMME patients and mucosal melanoma of other primary sites. Since mucosal melanomas are more frequent among Asian population, Lian et al. (21) compared the primary melanoma stage and patterns of metastases across anatomical sites in a large scale retrospective analysis. However, among 706 included patients, only 33 (5%) were from the upper GI tract (including esophagus, gastric and small bowel). Comparing with other primary sites, upper GI tract patients were more likely to have advanced stage (stage IV account for 45.5%) and have lymph node metastases. Moreover, all of them had multiple distant metastases at initial diagnosis and the prognosis of this entity was grave (5-year OS 4.2–20.0%). Another study focused on European population evaluated 86 mucosal melanoma patients over a period of 15 years also demonstrated that those with GI tract originated had a higher incidence of regional nodal metastases (62.5%) and T4 stage at diagnosis, and the mean OS time was only 16 months (22). These results were similar with our finding and further studies concerning the optimal treatment among PMME patients are warranted.
There are a few limitations of our study due to the SEER-based data collection. First, several variables, such as the recurrence date, nodal metastatic status, and systemic treatment modalities, including chemotherapy and targeted therapy, could not be obtained from SEER. Second, inherent biases exist in non-randomized and retrospective studies. In addition, 55 cases of PMME were identified in the SEER database. The staging category was available for only 47 of 55 cases; thus, the small sample size could lead to negative findings from the survival analysis.
In conclusion, PMME is an extremely rare disease with a dismal prognosis compared to other esophageal histological types. Most of the PMME patients in our cohort were female and Caucasian and had early stage disease. Female gender was an independent protective factor of PMME, and radical surgical resection might be an effective treatment option. More cases with adequate information are required to understand PMME more thoroughly.
Full table
Full table
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.10.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Institutional ethical approval and informed consent were waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Makuuchi H, Takubo K, Yanagisawa A, et al. Esophageal malignant melanoma: analysis of 134 cases collected by the Japan Esophageal Society. Esophagus 2015;12:158-69. [Crossref]
- Iwanuma Y, Tomita N, Amano T, et al. Current status of primary malignant melanoma of the esophagus: clinical features, pathology, management and prognosis. J Gastroenterol 2012;47:21-8. [Crossref] [PubMed]
- Baur E. Ein Fall von Primaerem Melanoma de Oesophagus. Arb Geb Pathol Anat Inst Tuebingen 1906;5:343-54.
- Chalkiadakis G, Wihlm JM, Morand G, et al. Primary malignant melanoma of the esophagus. Ann Thorac Surg 1985;39:472-75. [Crossref] [PubMed]
- Joob AW, Haines GK, Kies MS, et al. Primary malignant melanoma of the esophagus. Ann Thorac Surg 1995;60:217-22. [Crossref] [PubMed]
- Volpin E, Sauvanet A, Couvelard A, et al. Primary malignant melanoma of the esophagus: a case report and review of the literature. Dis Esophagus 2002;15:244-9. [Crossref] [PubMed]
- Bisceglia M, Perri F, Tucci A, et al. Primary malignant melanoma of the esophagus: a clinicopathologic study of a case with comprehensive literature review. Adv Anat Pathol 2011;18:235-52. [Crossref] [PubMed]
- Sabanathan S, Eng J, Pradhan GN. Primary malignant melanoma of the esophagus. Am J Gastroenterol 1989;84:1475-81. [PubMed]
- Maekawa T, Satoh K, Maekawa H, et al. A case of primary malignant melanoma arising in the esophagus. Nippon Gekakei Rengo Gakkai Zasshi 2005;30:154-9. [Crossref]
- Duggan MA, Anderson WF, Altekruse S, et al. The Surveillance, Epidemiology, and End Results (SEER) Program and Pathology: Toward Strengthening the Critical Relationship. Am J Surg Pathol 2016;40:e94-e102. [Crossref] [PubMed]
- Yamamoto S, Makuuchi H, Kumaki N, et al. A Long Surviving Case of Multiple Early Stage Primary Malignant Melanoma of the Esophagus and a Review of the Literature. Tokai J Exp Clin Med 2015;40:90-5. [PubMed]
- Lohmann CM, Hwu WJ, Iversen K, et al. Primary malignant melanoma of the oesophagus: a clinical and pathological study with emphasis on the immunophenotype of the tumours for melanocyte differentiation markers and cancer/testis antigens. Melanoma Res 2003;13:595-601. [Crossref] [PubMed]
- Sanchez AA, Wu TT, Prieto VG, et al. Comparison of primary and metastatic malignant melanoma of the esophagus: clinicopathologic review of 10 cases. Arch Pathol Lab Med 2008;132:1623-9. [PubMed]
- Taniyama K, Suzuki H, Sakuramachi S, et al. Amelanotic malignant melanoma of the esophagus: case report and review of the literature. Jpn J Clin Oncol 1990;20:286-95. [PubMed]
- Bohanes P, Yang D, Chhibar RS, et al. Influence of sex on the survival of patients with esophageal cancer. J Clin Oncol 2012;30:2265-72. [Crossref] [PubMed]
- Morita M, Otsu H, Kawano H, et al. Gender differences in prognosis after esophagectomy for esophageal cancer. Surg Today 2014;44:505-12. [Crossref] [PubMed]
- Wang Y, Hunt K, Nazareth I, et al. Do men consult less than women? An analysis of routinely collected UK general practice data. BMJ Open 2013;3:e003320 [Crossref] [PubMed]
- Sasaki K, Uchikado Y, Omoto I, et al. Multidisciplinary therapy for metastatic primary malignant melanoma of the esophagus: A case report. Mol Clin Oncol 2018;8:533-8. [PubMed]
- Sharabi AB, Lim M, DeWeese TL, et al. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol 2015;16:e498-509. [Crossref] [PubMed]
- Kirchberger MC, Ugurel S, Mangana J, et al. MEK inhibition may increase survival of NRAS-mutated melanoma patients treated with checkpoint blockade: Results of a retrospective multicentre analysis of 364 patients. Eur J Cancer 2018;98:10-6. [Crossref] [PubMed]
- Lian B, Cui CL, Zhou L, et al. The natural history and patterns of metastases from mucosal melanoma: an analysis of 706 prospectively-followed patients. Ann Oncol 2017;28:868-73. [PubMed]
- Cinotti E, Chevallier J, Labeille B, et al. Mucosal melanoma: clinical, histological and c-kit gene mutational profile of 86 French cases. J Eur Acad Dermatol Venereol 2017;31:1834-40. [Crossref] [PubMed]