
Knockdown of HOTAIR reduces the malignancy of bladder cancer cells via downregulation of invasions and metastasis-related genes
Introduction
Bladder cancer is one of the most common malignant tumors. The incidence of the cancer ranks fourth in men and tenth in women (1). The most common pathological type of bladder cancer is urothelial carcinoma, which may be non-musculoinvasive and musculoinvasive. After radical resection, about 50% of the musculocutaneous invasive bladder cancer would metastasize with a 5-year survival rate of 50% (2). To understand the mechanisms underlying the metastasis, the molecular aspects of cancer cells have been investigated. Long chain non-coding RNA (LncRNA) generally refers to RNA transcript exceeding 200 nt. Studies have shown that although they do not encode proteins, they regulate the expression of genes in various ways (3). LncRNA exerts different biological functions through various molecular mechanisms. For example, HOX transcript antisense intergenic RNA (HOTAIR) plays an important role in the proliferation of breast cancer cells (4). X-inactive specific transcript (Xist) inactivates chromosome X (5) and Nron regulates the nuclear function (6). LncRNA regulates gene expression and stabilizes cell morphology to impact the proliferation of cells. For instance, LncRNA may an oncogenic or tumor suppressor, and may bind to chromatin form complex to silence specific genes (7). HOTAIR is a long noncoding RNA that is transcribed from the antisense strand of the homeobox C gene locus in the chromosome 12. It is overexpressed in various carcinomas including breast cancer and colorectal cancer, suggesting that it is an oncogene (7-9). The regulatory site of the 2,158 nt long antisense LncRNA is not the HOX gene cluster where HOTAIR is encoded (10). In mammals, the HOX family is clustered in 4 different genomic positions (the HOXA, HOXB, HOXC, HOXD gene clusters). HOTAIR is located in the HOXC11 and HOXC12 loci in the human genome, and is transcribed at the HOXC site. HOTAIR can bind to two complexes at the same time, and then recruit them to specific genomic loci to silence the genes in the loci (8,11). Therefore, we studied the effect of silencing HOTAIR on the malignant differentiation, invasion and metastasis of bladder cancer in order to provide new clues for clinical treatment of bladder cancer.
Methods
Cell lines
Human bladder cancer lines 253J and T24 (Cat No. HY-DXT-209, HY-DXT-349) were purchased from the Cell Bank of the Chinese Academy of Science. Cells were maintained in DMEM medium containing 10% fetal bovine serum (FBS) at 37 °C in 5% CO2.
Reagents and instruments
DMEM (Cat No. KGM12400S-500) was purchased from KGI biologicals, Nanjing, China; FBS (Cat No. SKU:04-007-1A) was obtained from Biological Industries, Connecticut, USA; siHOTAIR and negative control were obtained from Shenggong, Shanghai, China; Trizon Reagent (Cat No. CW0580S), ultrapure RNA RNA extraction kit (Cat No. CW0581M) and HiFiScript first strand cDNA synthesis kit (Cat No. CW2569M) were purchased from CWBIO, Beijing, China; rabbit monoclonal antibodies against Notch 1 (Cat No. ab52627, 1:1,500 dilution), EpCAM (Cat No. ab32392, 1:6,000 dilution), E-cadherin (Cat No. ab40772, 1:5,000 dilution), vimentin (Cat No. ab92574, 1:3,500 dilution) were obtained from Abcam, Cambridge, USA; rabbit polyclonal antibodies against CXCR2 (Cat No. bs-12247R, 1:1,500 dilution) and MMP-2 (Cat No. bs-20706R, 1:600 dilution) were obtained from Bioss, Woburn, USA; rabbit monoclonal antibody against α-SMA (Cat No. M0002, 1:800 dilution) was purchased from Boster, Beijing, China. Vertical electrophoresis apparatus (Cat No. 0411312151351), ultrasensitive chemiluminescence imaging system (Chemi DocTM XRS+) and fluorescence PCR instrument (CFX Connect) were purchased from Bio-rad Laboratories, Shanghai, China. Fluorescence microscopy (IX51) was from Olympus, Kyoto, Japan.
Transfection
Cells were grown to 70% confluency, and transfected with lentivirus containing siHOTAIR (HOTAIR KD group), or negative control (Vector group). Non-transfected cells were used as control. For transfection, cells were grown to adhere to the wall, refreshed with new medium, and added with 0.5 µg/mL polybrene and lentivirus (200 µL at a MOI of 5). Twenty-four h after transfection, the culture medium was refreshed. When cells reached a confluency of 70%, they were harvested, washed twice with PBS and digested with 1% trypsin at 37 °C for 3 min. The digested cells were suspended in DMEM medium containing 10% FBS and cultured in the wells of 6-well plate as described above. The RNAi sequence and the negative control sequences were as follows:
- siHotair-1F: 5'-GATCCGCCTTTGGAAGCTCTTGAAGGCTCGAGCCTTCAAGAGCTTCCAAAGGC TTTTT G-3';
- siHotair-1R: 5'-AATTCAAAAAGCCTTTGGAAGCTCTTGAAGGCTCGAGCCTTCAAGAGCTTCCAAAGGC G-3';
- ShHOTAIR-2F: 5'-GATCCGAGAAGTGCTGCAACCTAAACCTCGAGGTTTAGGTTGCAGCACTTCTCTTTTTG-3';
- ShHOTAIR-2R: 5'-AATTCAAAAAGAGAAGTGCTGCAACCTAAACCTCGAGGTTTAGGTTGCAGCACTTCTCG-3';
- ShHOTAIR-3F: 5'-GATCCGCAACCTAAACCAGCAATTACCTCGAGGTAATTGCTGGTTTAGGTTGCTTTTTG-3';
- ShHOTAIR-3R: 5'-AATTCAAAAAGCAACCTAAACCAGCAATTACCTCGAGGTAATTGCTGGTTTAGGTTGCG-3'.
Negative control:
- Top strand 5'-GATCC TTCTCCGAACGTGTCACGTAATTCAAGAGATTACGTGACACGTTCGGAGAATTTTTTg-3';
- Bottom strand 5'-AATTC AAAAAATTCTCCGAACGTGTCACGTAATCTCTTGAATTACGTGACACGTTCGGAGAAg-3'.
CCK8 assay
CCK8 assays were preformed according to the supplier’s instruction. Briefly, cells were harvested, inoculated into the wells of 96-well plates, added with 10 µL CCK8 assay solution and cultured for 4 h. OD value was measured at 450 nm using microplate reader. The experiment was repeated 3 times, and the average value of the experimental results was taken as the final experiment result.
Flow cytometry
Cell apoptosis was assayed using Annexin V-PI assay according to the supplier’ instructions. Briefly, the cells were seeded in the wells of 6-well plates, grown to a confluency between 50% and 70%, and harvested for apoptosis assay after staining with Annexin V-PI for 30 min in the dark.
For cell cycle analysis, 1×106 cells were harvested, washed with PBS and fixed with ice-cold 70% ethanol overnight at 4 °C. Cells were washed with PBS and incubated with RNase A (100 µg/mL) at 37 °C for 30 min. DNA was labeled with PI (50 µg/mL) and the fluorescence was measured with a FACScalibur flow cytometer (Becton Dickinson). Data collection and analysis of the cell cycle distribution were performed using CellQuest and the Modfit software (Becton Dickinson).
Hoechst staining
Cells were fixed and stained with Hoechst 33258 for 5 min at room temperature. The stained cells were added with anti-fluorescence quenching solution and mounted to the slides, and reviewed under fluorescence microscope.
Fluorescent quantitative PCR
RNA was extracted using the TRIzon Reagent and reversely transcribed into cDNA using the HiFiScript first strand cDNA synthesis kit according to the manufacturer’s instructions. A total of 2.5 µL of the resultant cDNA was subjected to pre-amplification using the TaqMan Pre-Amp Master Mix (Applied Biosystems) in a total volume of 12 µL. Non-fluorescent probes were used at 1×. Pre-amplification cycling conditions were 10 min at 95 °C followed by 14 cycles, each one consisting of 15 s at 95 °C and 4 min at 60 °C. Later on, a 1:5 dilution of the pre-amplified cDNA was performed. RT-qPCR was performed on the 7900HT Fast Real-Time PCR system using TaqMan gene expression assays probes (Applied Biosystems). Human glyceraldehyde-3-phosphate dehydrogenase, GADPH (Hs03929097_g1), was used as an internal control. The primers used were as follows:
HOTAIR F: 5'- CTTGCTCTTCTTATCATCTCCATC, HOTAIR R: 5'- GAACCCTCTGACATTTGCCTA; Notch1 F: 5'-CTTTGTGCTTCTGTTCTTCGTG, Notch1 R: 5'-CGCCGCTTCTTCTTGCT; EpCAM F: 5’-TTCGGGCTTCTGCTTGC, EpCAM R: 5'-CCCTTCAGGTTTTGCTCTTC; CXCR2 F: 5'-ATGTCTCAGCATCTGGGGTCT, CXCR2 R: 5'-GCAGGGTGAATCCGTAGCA; E-cadherin F: 5'-ATCTGAAAGCGGCTGATACTG, E-cadherin R: 5'-TGCCCCATTCGTTCAAGTA; MMP-2 F: 5'-CCGCAGTGACGGAAAGA, MMP-2 R: 5'-TGGTGTAGGTGTAAATGGGTG; Vimentin F: 5'-TCGTGATGCTGAGAAGTTTCG, Vimentin R: 5'-TCTGGATTCACTCCCTCTGGT; α-SMA F: 5'-GCGTGGCTATTCCTTCGT, α-SMA R: 5'-TCAGGCAACTCGTAACTCTTCT; GAPDH F: 5'-CAATGACCCCTTCATTGACC, GAPDH R: 5'-GAGAAGCTTCCCGTTCTCAG.
The data were managed using the Applied Biosystems software RQ Manager v1.2.1. Relative expression was calculated by using the comparative Ct method and obtaining the fold change value (2−ΔΔCt) according to previously described protocol (12).
Western blot analysis
Cells of 2×107 were washed twice with cold PBS and lysed with RIPA buffer that contains protease and phosphatase inhibitors cocktail (Roche, UK). The supernatants were collected after centrifugation at 12,000 rpm for 20 min. The protein was subjected to polyacrylamide gel electrophoresis (SDS-PAGE), transferred to a PVDF membrane, and then detected by the proper primary and secondary antibodies before visualization with a chemiluminescence kit. The intensity of blot signals was quantitated using Quantity One software (v.4.62, General Electric, UK).
Statistical analysis
All data were expressed as mean ± standard error of the mean (SEM) obtained from at least three independent experiments. Statistical comparisons between experimental and control groups were assessed by using the Student’s t-test. P<0.05 was considered statistically significant.
Results
siHOTAIR inference
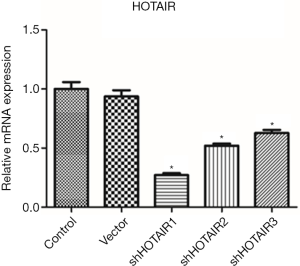
Human bladder cancer 253J cells were transfected with three siHOTAIRs. Quantification of mRNA showed that HOTAIR levels were significantly down-regulated compared with control and cells transfected with negative control (P<0.05, Figure 1). Among them shHOTAIR1 had the greatest silencing effect, while there was no difference between the control and vector groups, indicating that the gene was silenced by siHOTAIRs specifically. Since siHOTAIR1 was most effective, it was used in subsequent experiments.
siHOTAIR reduced cell proliferation
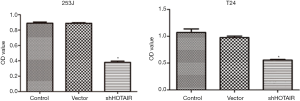
We then measured the proliferation of 253J and T24 cells after siHOTAIR transfection. As shown in Figure 2, compared with the control and vector-transfected cells, the OD values of siHOTAIR-transfected cells were significantly decreased (P<0.05), while there was no difference between control and vector-transfected cells, indicating that the proliferation of cells was specifically inhibited by siHOTAIR.
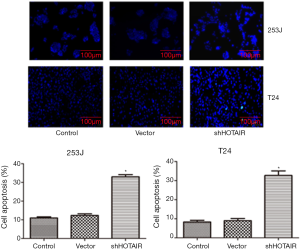
siHOTAIR increased apoptosis
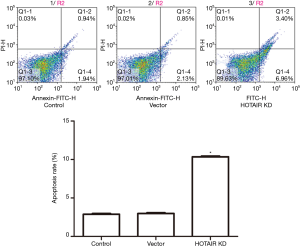
Flow cytometry showed that the percentage of apoptotic cells increased significantly after the cells were transfected with siHOTAIR as compared with the control and vector (P<0.05, Figure 3), while these percentages were the same between the control and vector groups. This was further confirmed using Hoechst staining (Figure 4).
siHOTAIR arrested cells in S stage
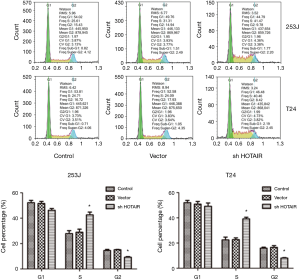
After siHOTAIR transfection, more cells were observed in S stage and less in G1 stage in both 253 and T24 cell lines (Figure 5).
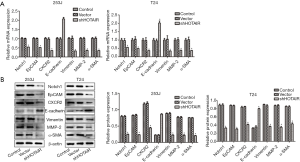
Impact of siHOTAIR on gene expression
We then assayed the expression of genes related to invasions and metastasis of cancer in the cells. The results showed that the mRNA and protein levels of Notch1, EpCAM, CXCR2, E-cadherin, vimentin, MMP-2 and α-SMA were significantly down-regulated, while these of E-cadherin were significantly up-regulated after the cells were transfected with siHOTAIR. The expression of these genes was not affected after transfection with vector at mRNA and protein levels (Figure 6).
Discussion
Bladder cancer is one of the most common malignant tumors in the urinary system. It has the biological characteristics of easy recurrence, easy invasion and frequent metastasis. Gupta et al. showed that enforced expression of HOTAIR in epithelial cancer cells induced genome-wide re-targeting of Polycomb repressive complex 2 (PRC2) in an occupancy pattern like embryonic fibroblasts. This pattern leads to altered methylation and gene expression of histone H3, and increased cancer invasiveness (which the loss of HOTAIR can inhibit) and metastasis in a manner dependent on PRC2 (8). Chen et al. found that HOTAIR is closely related to the metastasis of squamous cell carcinoma of the esophagus (13). In the primary and metastatic tissues of breast cancer, HOTAIR is highly expressed (8) and the high expression of HOTAIR is closely related to the metastasis and recurrence of liver cancer and the drug resistance of hepatoma cells (10). In addition, a significant inverse correlation between HOTAIR and WIF-1 expression was demonstrated in Ta/T1 BC tissues and upregulation of HOTAIR predicts recurrence in stage Ta/T1 bladder cancer (14). After the cells were transfected with siHOTAIR, the viability of cells decreased significantly and the apoptosis was promoted (Figures 2-4), demonstrating that the proliferation of bladder cancer cells could be inhibited after the down-regulation of HOTAIR. This is consistent with the previous results (8).
The NOTCH gene was first discovered in 1917 in Drosophila melanogaster. It is so named because due to its absence, the fly’s wings have the notch if the gene is absent. The gene widely exists and is expressed in a variety of organisms (15). The Notch signaling pathway is highly conserved in most multicellular organisms and plays an important role in cell differentiation and embryo development. The signaling pathway also impacts cell apoptosis, proliferation, cell cycle, differentiation, under different physiological and pathological conditions (16). Therefore, we investigated the effect of HOTAIR silence on the expression of genes in the pathways. Notch1 decreased after down-regulating HOTAIR expression, suggesting that HOTAIR may affect the apoptosis through down-regulating the expression of Notch1. We also investigated how the expression of EpCAM, EpCAM stimulates the expression of proto-oncogenes such as cyclin A/E and c-myc to induce tumors such as colon carcinoma (17) and is highly expressed in many epithelial tumors, such as squamous cell carcinoma (18) and breast cancer (19). Therefore, it can be used as a candidate protein for tumor diagnosis and treatment. The expression of EpCAM also decreased after down-regulating HOTAIR in our study. This might suppress the expression of some proto oncogenes and inhibit the growth of tumor cells as we observed.
E-cadherin is a calcium-dependent cell adhesion molecule. It maintains the integrity of cell by binding to identical proteins in adjacent cells. Reduced expression of E-cadherin is an indicator of epithelial-mesenchymal transition (EMT) (20). Vimentin is an intermediate filament protein. Its functions include the maintenance of cell morphology, signal transduction, transplantation immunity and apoptosis (21). α-SMA regulates cell movement. The expression of α-SMA promotes EMT (22), which is mainly involved in growth and development, injury repair, tumor metastasis and the occurrence and development of fibrosis diseases. When HOTAIR was down-regulated, the expression of E-cadherin was up-regulated while the expression of Vimentin and α-SMA was down-regulated. These results suggest that the biological characteristics of bladder cancer may be affected by HOTAIR silence as a result of changed expression of E-cadherin, vimentin and α-SMA.
The chemokine CXC family has 5 receptors, such as CXCR1 and CXCR2. They are overexpressed in breast cancer, colon cancer, bladder cancer and other tumor cells (23). Knockdown of HOTAIR in UBC cell lines reduces in vitro migration and invasion. Importantly, loss of HOTAIR expression in UBC cell lines alters expression of EMT genes (24). Our results show that after the downregulation of HOTAIR, the expression of CXCR2 decreased. This might contribute to reduced growth of tumor cells after HOTAIR downregulation. MMPs are zinc-dependent endopeptidases, which degrade the extracellular matrix, effectively leading to tumor invasion and metastasis (25). MMP-2 is one of the most important subtypes of MMPs and is highly expressed in breast cancer, ovarian tumor and nasopharyngeal carcinoma (26). Its expression in the tumor cells was significantly decreased after the cells were transfected with siHOTAIR, suggesting that HOTAIR is related to the biological characteristics of the malignant differentiation in bladder cancer.
Conclusions
siHOTAIR can reduce the expression of HOTAIR, resulting in reduced cell growth and apoptosis. A number of pro oncogenes are up-regulated as a result of HOTAIR down-regulation, providing possible molecular mechanism underlying the inhibition. These results further our understanding of the metastasis of bladder cancer and could help provide new clues for the diagnosis and treatment of bladder cancer.
Acknowledgments
Funding: This study was supported by Grants-in-Aid for Scientific Research from Central Hospital, Suining, China (grant number: 2015y21).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.10.03). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Institutional ethical approval and informed consent were waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893-917. [Crossref] [PubMed]
- Kim WJ, Kim EJ, Kim SK, et al. Predictive value of progression-related gene classifier in primary non-muscle invasive bladder cancer. Mol Cancer 2010;9:3. [Crossref] [PubMed]
- Caley DP, Pink RC, Trujillano D, et al. Long noncoding RNAs, chromatin, and development. ScientificWorldJournal 2010;10:90-102. [Crossref] [PubMed]
- Berteaux N, Lottin S, Monte D, et al. H19 mRNA-like noncoding RNA promotes breast cancer cell proliferation through positive control by E2F1. J Biol Chem 2005;280:29625-36. [Crossref] [PubMed]
- Brown CJ, Lafreniere RG, Powers VE, et al. Localization of the X inactivation centre on the human X chromosome in Xq13. Nature 1991;349:82-4. [Crossref] [PubMed]
- Willingham AT, Orth AP, Batalov S, et al. A strategy for probing the function of noncoding RNAs finds a repressor of NFAT. Science 2005;309:1570-3. [Crossref] [PubMed]
- Reis EM, Verjovski-Almeida S. Perspectives of Long Non-Coding RNAs in Cancer Diagnostics. Front Genet 2012;3:32. [Crossref] [PubMed]
- Gupta RA, Shah N, Wang KC, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature 2010;464:1071-6. [Crossref] [PubMed]
- Kogo R, Shimamura T, Mimori K, et al. Long noncoding RNA HOTAIR regulates polycomb-dependent chromatin modification and is associated with poor prognosis in colorectal cancers. Cancer Res 2011;71:6320-6. [Crossref] [PubMed]
- Yang Z, Zhou L, Wu LM, et al. Overexpression of long non-coding RNA HOTAIR predicts tumor recurrence in hepatocellular carcinoma patients following liver transplantation. Ann Surg Oncol 2011;18:1243-50. [Crossref] [PubMed]
- Rinn JL, Kertesz M, Wang JK, et al. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell 2007;129:1311-23. [Crossref] [PubMed]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001;25:402-8. [Crossref] [PubMed]
- Chen FJ, Sun M, Li SQ, et al. Upregulation of the long non-coding RNA HOTAIR promotes esophageal squamous cell carcinoma metastasis and poor prognosis. Mol Carcinog 2013;52:908-15. [Crossref] [PubMed]
- Yan TH, Lu SW, Huang YQ, et al. Upregulation of the long noncoding RNA HOTAIR predicts recurrence in stage Ta/T1 bladder cancer. Tumour Biol 2014;35:10249-57. [Crossref] [PubMed]
- Fouladi M, Stewart CF, Olson J, et al. Phase I trial of MK-0752 in children with refractory CNS malignancies: a pediatric brain tumor consortium study. J Clin Oncol 2011;29:3529-34. [Crossref] [PubMed]
- Yamamoto S, Schulze KL, Bellen HJ. Introduction to Notch signaling. Methods Mol Biol 2014;1187:1-14. [Crossref] [PubMed]
- Maetzel D, Denzel S, Mack B, et al. Nuclear signalling by tumour-associated antigen EpCAM. Nat Cell Biol 2009;11:162-71. [Crossref] [PubMed]
- Stoecklein NH, Siegmund A, Scheunemann P, et al. Ep-CAM expression in squamous cell carcinoma of the esophagus: a potential therapeutic target and prognostic marker. BMC Cancer 2006;6:165. [Crossref] [PubMed]
- Osta WA, Chen Y, Mikhitarian K, et al. EpCAM is overexpressed in breast cancer and is a potential target for breast cancer gene therapy. Cancer Res 2004;64:5818-24. [Crossref] [PubMed]
- Yue B, Qiu S, Zhao S, et al. LncRNA-ATB mediated E-cadherin repression promotes the progression of colon cancer and predicts poor prognosis. J Gastroenterol Hepatol 2016;31:595-603. [Crossref] [PubMed]
- Dong Q, Zhu X, Dai C, et al. Osteopontin promotes epithelial-mesenchymal transition of hepatocellular carcinoma through regulating vimentin. Oncotarget 2016;7:12997-3012. [Crossref] [PubMed]
- Yan D, Gu X, Jiang S, et al. mRNA-binding protein Human-antigen R regulates alpha-SMA expression in human bronchia smooth muscle cells. Zhonghua Yi Xue Za Zhi 2015;95:3147-9. [PubMed]
- Wang J, Hu W, Wang K, et al. Repertaxin, an inhibitor of the chemokine receptors CXCR1 and CXCR2, inhibits malignant behavior of human gastric cancer MKN45 cells in vitro and in vivo and enhances efficacy of 5-fluorouracil. Int J Oncol 2016;48:1341-52. [Crossref] [PubMed]
- Berrondo C, Flax J, Kucherov V, et al. Expression of the Long Non-Coding RNA HOTAIR Correlates with Disease Progression in Bladder Cancer and Is Contained in Bladder Cancer Patient Urinary Exosomes. PLoS One 2016;11:e0147236 [Crossref] [PubMed]
- Banerji A, Chakraborti J, Mitra A, et al. Cell membrane-associated MT1-MMP-dependent activation of pro-MMP-2 in A375 melanoma cells. J Environ Pathol Toxicol Oncol 2005;24:3-17. [Crossref] [PubMed]
- Che YL, Luo SJ, Li G, et al. The C3G/Rap1 pathway promotes secretion of MMP-2 and MMP-9 and is involved in serous ovarian cancer metastasis. Cancer Lett 2015;359:241-9. [Crossref] [PubMed]


