In-vitro evaluation of HSP27 inhibitors function through HER2 pathway for ovarian cancer therapy
Introduction
Ovarian cancer starts as a single tumor, then mutates to a heterogeneous complex disease that is composed of different types of tumors (1). It remains the most fatal disease in women reproductive system. Even if the disease is in the early stages, early detection is sometimes difficult. Although the standard approved chemotherapy treatments for ovarian epithelial, fallopian, and peritoneal cancers are bevacizumab (Avastin), Alkeran (Melphalan), Bevacizumab, Carboplatin, Cisplatin and Olaparib (Lynparza) in a single or in a combination form, sometimes show undesirable drug resistance after the initial promising treatment. Therefore, the development of new drugs is required to enhance the drug pharmacological activity and to reduce the drug’s side effects based on reliable protein biomarkers. This will effectively help in ovarian cancer early detection and treatment. Kim et al. and others have stated that HSP27, and HER2 are both reliable cancer biomarkers (2-7).
HSP27 is a low molecular weight protein (27 kDa), which is expressed in all eukaryotic cells. It plays a role in normal cell proliferation, differentiation, invasion, metastasis, and death. HSP27 exists in two forms: either as a large oligomer for chaperon intrinsic functions or as a small oligomer that binds to the microfilaments to stabilize them as extrinsic functions Figure 1A (6,8). It is also known to assist the correct folding of the misfolded proteins to the correct chain structure (9,10). In addition, HSP27 prevents apoptosis through inhibition of apoptotic protein release from the mitochondria. It is a group of polypeptides, which work when the cell is subjected to stressful conditions such as an increase in temperature or pressure, or when the cell is faced with some toxic compounds such as chemotherapy drugs (11). Stress induces an increase of expression and phosphorylation of HSP27. HSP27 interacts with many proteins to stabilize them and prevents a variety of chemotherapy drugs from causing cancer cell death (12). Thus, clinically HSP27 displays drug resistance to chemotherapy treatment in some types of cancers, and there have been reports stating that high HSP27 expression is associated with poor patient survival rates (2,4-6). In addition, HSP27 over-expression was found in a number of malignant cancer types such as prostate, breast, pancreatic, and ovarian cancers (13). Further, HSP27 is over-expressed in a late stage ovarian malignant tumor more than the early stage of the benign tumor. Although the cellular role of HSP27 in cancers is not yet completely understood, HSP27 is potentially a good molecular target for cancer therapy (5,6).
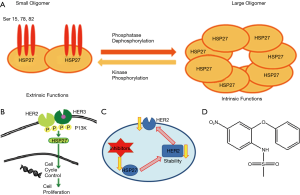
HER2, on the other hand is a trans-membrane protein tyrosine kinase receptor, which regulates cell survival, proliferation, differentiation, and migration. Over-expression or mutation of HER2 is associated with a malignant phenotype in many cancers, including ovarian cancer (14,15). This protein forms a dimer by binding to another family member growth factor receptor in the cell membrane, which activates downstream signaling pathway through kinase phosphorylation; then this process activates HSP27 protein’s function, which is important for the cell cycle check points Figure 1B (5). Studies have shown that HSP27 expression is directly proportional to HER2 expression up/down regulation and both are regulated through MAPK phosphorylation pathway (14,16-18). There are many drugs of monoclonal antibodies that target HER2, the common drug used being trastuzumab (Herceptin). It is a humanized recombinant monoclonal antibody, which is used clinically to treat patients with HER2 positive (5,19,20). HER2 over-expression cell lines were down-regulated after treatment with herceptin such as SKBR3 breast cancer cell line (2). However, the majority of patients that initially respond to herceptin begin to progress again within one year (20). In addition, HSP27 has been recognized to reduce cellular herceptin’s ability by direct binding to HER2 and increasing its stability (18,21). HER2 over-expression ovarian cancer is more resistant to the chemotherapy drugs including taxol, which leads to aggressive progression in ovarian cancer (19,20).
HSP27 and HER2 interactions’ complex often increases the protein stability and up regulated HSP27 protein expression levels, which have been reported in HER2 positive breast tumors Figure 1C (21,22). In order to overcome the barrier of chemotherapy drug resistance, nimesulide drug was used as a starting material to synthesize a series of compounds for the inhibition of HSP27 function (23-25). Nimesulide is a non-steroidal, anti-inflammatory drug, and is characterized by analgesic, antipyretic, and anti-proliferation properties Figure 1D.
The main aim of this study is to find HER2 over-expression ovarian cancer cell line (OVCAR3, SKOV3, & HEY1B) using western blot assay, followed by the screening and evaluation of the potent anticancer agents’ activities using the standard MTT assay designed to assess the cell proliferation after HSP27 suppression. It is worth noting that our goal is to target dual proteins through HER2 pathway Figure 1C (26-29).
Methods
Cell lines and reagents
The human ovarian cancer cell lines SKOV3, OVCAR3 and HEY1B were obtained from the laboratory of Dr. Aimin Zhou at Cleveland State University. The anti-bodies (HSP27, HER2, HRP, B-actin) were purchased from Cell Signaling Technology. Cell culture media RPMI and other supplements were obtained from Thermo Fisher Scientific. Nimesulide analogs were synthesized in Cleveland State University laboratory. MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide) is from Sigma-Aldrich (Milwaukee, WI). Chemicals and reagents including RIPA, PI, EDTA, SDS, protogel, protogel buffer, loading and sample buffers, Tween 20, glycine, TRIS, SDS, methanol, and Luminal are commercially available, and are ready for direct use without preparation.
Cell culture
The cells were maintained in RPMI1640 medium containing 10% FBS, and 10 mL penicillin-streptomycin and 100 µL ciprofloxacin. FBS was deactivated for 30 min in a 37 °C water bath before use. Cell cultures were grown at 37 °C, in a humidified atmosphere of 5% CO2 incubator.
Western blot assay
Confluent ovarian cancer cells dishes (OVCAR3-SKOV3-HEY1B) were washed with PBS and the cells were harvested by scraper. This was followed by re-suspension of the cells with PBS and then centrifuging the collected sample at 3,500 g. The supernatant were then removed and the collected cell pellets were lysed by mixing them with RIPA, PI, and EDTA 100 µL. The cells were then vortexed and centrifuged for 60 minutes at 16,500 g. Each cell line proteins were boiled with (2×) loading buffer for 5 min and then normalized and electrophoresed on a 10% SDS-polyacrylamide gel. The gel containing the proteins was transferred to nitrocellulose membrane followed by blocking of the non-specific proteins with 5% NFDM in PBST. The membranes were rinsed three times for five minutes with PBST (0.1% Tween 20). Then, the membrane was incubated with the primary antibody (target-HER2) for one hour (rabbit 1:1,000 dilution). Next, the membranes were washed three times with PBST for 10 minutes each and incubated with the secondary antibody (anti rabbit-HRP conjugated secondary antibody 1:1,000 dilution) for one hour. Similar steps repeated for the membrane incubated with HSP27 (rabbit 1:500 dilution) followed by one-hour incubation with the secondary antibody. Then, the membrane was again washed with PBST three times for 10 minutes each to be prepared for the visualization of the bands by chemiluminescence substrate. B-actin (1:1,000 dilution) was used as housekeeping control.
MTT assay
MTT assay was performed by monitoring the reduction of yellow MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) to violet color product. After culturing the cells in the 2D monolayer cell culture, cells were harvested and then seeded with RPMI1640 medium into 96-well, and incubated overnight. Then, cells were treated with different concentrations of anticancer agents in four replicates each and incubated for 72 hr. DMSO control wells received concentrations equal to those in the drug-treated cell wells. The cell viability was determined by MTT reagent the popular metabolic dye 100 µL of 0.5 mg/mL in fresh medium, added after the removal of the old medium and the cells were incubated for 1 hr. Supernatants were removed from the wells and 100 µL DMSO were added on the reduced MTT dye. The final absorbance measurement was determined using a plate reader at 570 nm and the values were normalized to controls.
Statistical analysis
Graph Pad Prism software (Graph Pad Software Incorporated) and Microsoft Excel (Microsoft Corporation) were used to determine the statistical and graphical information. IC50 values were normalized using nonlinear regression analysis.
Results and discussion
Determination of HER2 over-expression ovarian cancer cell line using western blot assay
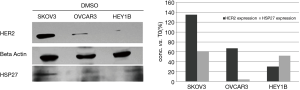
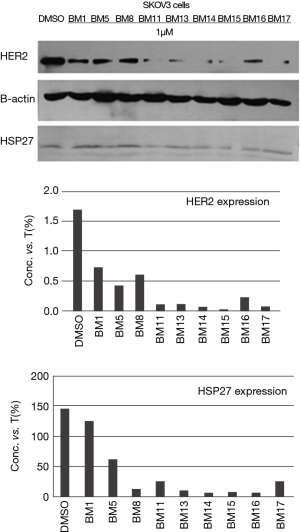
Western-blot analysis was performed as described previously. Cell lysates were collected from all three cancer cell lines to examine HER2 over-expression cell line. From the visualized bands, we found that SKOV3 cell line is highly expressing HER2 protein more than OVCAR3 and HEY1B cell lines Figure 2. Therefore, SKOV3 carcinoma cells, which express high HER2, treated with the potent selective compounds BM 1, 5, 8, 11, 13, 14, 15, 16, 17 at 1 µM concentration Figure 3. The anticancer agents showed potent reduction in HER2 expression compared with the DMSO control band. In depth, SKOV3 cells’ bands treated with the compounds BM 11, 13, 14, 15, 16, and 17 were much more potent than BM1, 5, and 8 in reducing HER2 protein expression.
Evaluation of HSP27 inhibitors treated on SKOV3, OVCAR3, & HEY1B ovarian cancer cell lines using MTT assay
HSP27 inhibitors were examined for their potency and selectivity on cell growth inhibition with SKOV3, OVCAR3 & HEY1B ovarian cancer cell lines using MTT assay. The IC50 values for each anti-cancer agent were summarized in Table 1. The data revealed that SKOV3 cell growth inhibition ranged from 0.010 to 0.039 µM. While, OVCAR3 cell line showed cell growth inhibition ranges from 0.052 to 5.989 µM. Also, HEY1B cell growth inhibition ranged from 0.013 to 208.8 µM. The data analysis showed that our agents have potent anti-cancer activity and almost all of the agents showed activity with SKOV3 cell line but some of the agents have potential only towards OVCAR3, and others only towards HEY1B cell line. However, the anticancer agents BM 1, 5, 8, 11, 13, 14, 15, 16, 17 had the potential towards both OVCAR3 and HEY1B cell lines, and selectively towards SKOV3 cell line, which is highly adapted cell growth inhibition Figure 3. Further in depth, analysis of the data revealed that BM 11, 13, 14, 15, 16 &17 were more potent compounds when treated with SKOV3, than BM 1, 5 and 8. This potency is related to the presence of the shared propyl side chain in the chemical structure Figure 4A. Further, in comparing the most potent compounds BM13 and BM17 (IC50 values and structures), their strengths related to the methoxy substituent on the benzene ring Figure 4B. In sum, all agents showed potent anticancer activity Figure 4C,D.
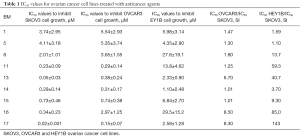
Full table

Conclusions
HSP27 and HER2 over-expression in ovarian carcinoma is still a matter of debate. Our anticancer agents were synthesized to target HSP27 protein function and decrease HER2 receptor’s stability through HER2 pathway. Interestingly, western blot assay data showed parallel results to the MTT assay’s data. Western blot analysis revealed that HER2 over-expression cell line is SKOV3 cell line, and the synthesized agents showed great anticancer activity towards it. In conclusion, suppression of HSP27 by anti-cancer agents reduced its function and decreased HER2 stability through HER2 pathway found in SKOV3 cells. The MTT assay data also revealed that SKOV3 cell line adapted cell growth inhibition more than OVCAR3 and HEY1B cell lines. Overall, our findings indicated that down regulation of HSP27 in SKOV3 cells lead to reduction of HER2 stability. These preliminary data indicated that we can synthesize novel anti-cancer agents, which are effective in targeting two proteins in cancer rather than operating only on a single protein.
Acknowledgments
Thanks due to Dr. Bin Su and Aimin Zhou, Cleveland State University, for providing ovarian cancer cell lines and the instruments. Also, research facilities provided by Chemistry Department, Kuwait University are gratefully acknowledged especially: instrument project no: GS01/03 and No: GS01/05, and No: GS02/01.
Funding: This project was supported by Kuwait University grant (ZS06/17).
Footnote
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.11.14). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Institutional ethical approval and informed consent were waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kanninen TT, Nasioudis D, Sisti G, et al. Epidemiology of Second Primary Tumors in Women With Ovarian Cancer. Int J Gynecol Cancer 2017;27:659-67. [Crossref] [PubMed]
- Kim MK, Kim SC, Kim WK, et al. HSP27 phosphorylation inhibitor regulates Her2 expression in human breast cancer cell line SK-BR-3 with induced Herceptin resistance. EPMA J 2014;5:A47. [Crossref]
- Cloven N, Kyshtoobayeva A, Burger R, et al. In vitro chemoresistance and biomarker profiles are unique for histologic subtypes of epithelial ovarian cancer. Gynecol Oncol 2004;92:160-6. [Crossref] [PubMed]
- Durán MC, Vega F, Moreno-Bueno G, et al. Characterisation of tumoral markers correlated with ErbB2 (HER2/Neu) overexpression and metastasis in breast cancer. Proteomics Clin Appl 2008;2:1313-26. [Crossref] [PubMed]
- Kang SH, Kang KW, Kim KH, et al. Upregulated HSP27 in human breast cancer cells reduces Herceptin susceptibility by increasing Her2 protein stability. BMC Cancer 2008;8:286. [Crossref] [PubMed]
- Stope M, Klinkmann G, Diesing K, et al. Heat Shock Protein HSP27 Secretion by Ovarian Cancer Cells Is Linked to Intracellular Expression Levels, Occurs Independently of the Endoplasmic Reticulum Pathway and HSP27's Phosphorylation Status, and Is Mediated by Exosome Liberation. Dis Markers 2017;2017:1575374 [Crossref] [PubMed]
- Vidyasagar A, Wilson N, Djamali A. Heat shock protein 27 (HSP27): biomarker of disease and therapeutic target. Fibrogenesis Tissue Repair 2012;5:7. [Crossref] [PubMed]
- Bitar K, Ibitayo A, Patil S. HSP27 modulates agonist-induced association of translocated RhoA and PKC-a in muscle cells of the colon. J Appl Physiol 1985;2002:41-9.
- Ciocca DR, Calderwood SK. Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones 2005;10:86-103. [Crossref] [PubMed]
- Kostenko S, Moens U. Heat shock protein 27 phosphorylation: kinases, phosphatases, functions and pathology. Cell Mol Life Sci 2009;66:3289-307. [Crossref] [PubMed]
- Schmitt E, Gehrmann M, Brunet M, et al. Intracellular and extracellular functions of heat shock proteins: repercussions in cancer therapy. J Leukoc Biol 2007;81:15-27. [Crossref] [PubMed]
- Kuramitsu Y. Is HSP27 a Key Molecule or a Biomarker of Cancers? J Gastrointest Dig Syst 2012;2:e106 [Crossref]
- Henriette JG, Hollema H, Lemstra W, et al. Heat-shock-protein-27(HSP27) expression in ovarian carcinoma: Relation in response to chemotherapy and prognosis. Int J Cancer 1999;84:234-8. [Crossref] [PubMed]
- Lanitis E, Dangaj D, Hagemann I, et al. Primary Human Ovarian Epithelial Cancer Cells Broadly Express HER2 at Immunologically-Detectable Levels. PLoS One 2012;7:e49829 [Crossref] [PubMed]
- Pickl M, Ries CH. Comparison of 3D and 2D tumor models reveals enhanced HER2 activation in 3D associated with an increased response to trastuzumab. Oncogene 2009;28:461-8. [Crossref] [PubMed]
- Wilken J, Webster K, Maihle N. Trastuzumab sensitizes ovarian cancer cells to EGFR-targeted therapeutics. J Ovarian Res 2010;3:7. [Crossref] [PubMed]
- Al Amri W, Balachandran I, Thangirala S, et al. Differential Association between HER2/neu and Angiogenesis in Breast Cancer. Am J Cancer Biol 2013;1:15.
- Khabele D, Kabir S, Dong Y, et al. Preferential Effect of Akt2-Dependent Signaling on the Cellular Viability of Ovarian Cancer Cells in Response to EGF. J Cancer 2014;5:670-8. [Crossref] [PubMed]
- Rani S, Corcoran C, Shiels L, et al. Neuromedin U: A Candidate Biomarker and Therapeutic Target to Predict and Overcome Resistance to HER-Tyrosine Kinase Inhibitors. Cancer Res 2014;74:3821-33. [Crossref] [PubMed]
- Amler LC, Wang Y, Hampton G. HER2 as a Therapeutic Target in Ovarian Cancer. Available online: https://www.semanticscholar.org/paper/HER2-as-a-Therapeutic-Target-in-Ovarian-Cancer-Amler-Wang/0d939bb61ff80afcab7c999380f1536beab2f314
- Song X, Kaivl S, Hu J, et al. Suppression of human epidermal growth factor receptor 2 via interference increases the chemosensitivity of ovarian carcinoma. Oncol Lett 2016;11:3028-32. [Crossref] [PubMed]
- DeFazio-Eli L, Strommen K, Dao-Pick T, et al. Quantitative assays for the measurement of HER1-HER2 heterodimerization and phosphorylation in cell lines and breast tumors: applications for diagnostics and targeted drug mechanism of action. Breast Cancer Res 2011;13:R44. [Crossref] [PubMed]
- Magnifico A, Albano L, Campaner S, et al. Tumor-initiating cells of HER2-positive carcinoma cell lines express the highest oncoprotein levels and are sensitive to trastuzumab. Clin Cancer Res 2009;15:2010-21. [Crossref] [PubMed]
- Smith JA, Ngo H, Martin MC, et al. An evaluation of cytotoxicity of the taxane and platinum agents combination treatment in a panel of human ovarian carcinoma cell lines. Gynecol Oncol 2005;98:141-5. [Crossref] [PubMed]
- Arrigo AP. Hsp27: novel regulator of intracellular redox state. IUBMB Life 2001;52:303-7. [Crossref] [PubMed]
- Kappé G, Verschuure P, Philipsen R, et al. Characterization of two novel human small heat shock proteins: protein kinase-related HspB8 and testis-specific HspB9. Biochim Biophys Acta 2001;1520:1-6. [Crossref] [PubMed]
- Yi X, Zhong B, Smith K, et al. Identification of a Class of Novel Tubulin Inhibitors. J Med Chem 2012;55:3425-35. [Crossref] [PubMed]
- Zhong B, Chennamaneni S, Lama R, et al. Synthesis and anticancer mechanism investigation of dual Hsp27 and tubulin inhibitors. J Med Chem 2013;56:5306-26. [Crossref] [PubMed]
- Zhong B, Lama R, Kulman D, et al. Lead optimization of dual tubulin and Hsp27 inhibitors. Eur J Med Chem 2014;80:243-53. [Crossref] [PubMed]