
AZT sensitizes hepatocellular carcinoma cells to As2O3 by up-regulating the arsenic transporter aquaglyceroporin 9
Introduction
Arsenic trioxide (As2O3) is a highly efficacious drug (1) and has been shown to be successful as a first-line treatment option in induction therapy for the patients with acute promyelocytic leukemia (APL) and as a maintenance therapy (2,3). Furthermore, As2O3 has also been reported to have anti-proliferative and proapoptotic activities in some solid tumors, including lung adenocarcinoma, hepatocellular carcinoma and cervical cancer (4-6). As a drug that has chemo-preventative qualities, As2O3 has been demonstrated to induce apoptosis through the generation of a reactive oxygen species and a superoxide compound (7,8). This leads to cell arrest through the processes of demethylation and alteration in the expression level of the cell cycle-related genes leading to cell cycle arrest (9), induction DNA damage, and the disruption of the mitochondrial transmembrane potential with the release of cytochrome c and caspase activation (10). However, As2O3 is a double-edged sword during cancer treatment. Its cardiotoxicity, neurotoxicity and other side effects (11-14) are a significant obstacle for further application. Side effects such as cardiotoxicity and neurotoxicity present a major obstacle for its use in a clinical environment.
3'-azido-3'-deoxythymidine (AZT) is a thymidine analogue, which has been shown to have antitumor effects against adult T-cell leukemia/lymphoma and pancreatic cancers. As Namba reported, AZT could resensitize the gemcitabine-resistant pancreatic cancer cells for gemcitabine via the inhibition of the Akt-GSK3β-Snail pathway (15). Accordingly, our previous results showed that As2O3 combined with AZT has significantly important synergistic inhibitory effects on the proliferation, migration and the invasion abilities of the HepG2 cells (16,17). However, the mechanism is still unknown.
Since As2O3 shows its cytotoxic activity only when entering cells, the arsenic carrier appeared to be an important player for modulating arsenic sensitivity. Previous studies have shown that aquaglyceroporin 9 (AQP9), which is mostly expressed in tumor cells and human leukocytes, was supposed to be related to the absorption of As2O3. This indicated that it has a critical role in determining the sensitivity of the tumor cells towards the As2O3 induced cytotoxicity (18-20). In acute myeloid leukemia cells, azacytidine facilitates the uptake of As2O3 by up-regulating the arsenic transporter AQP9 (21). Moreover, all-trans retinoic acid (ATRA) up-regulated AQP9, contributes to a significantly increased As2O3-induced cytotoxicity on combination with As2O3 (20). Chen and his colleagues found that AQP9 played a key role in the proliferation, apoptosis and metastasis of androgen-independent prostate cancer cells (22), which shows the same effects with our previous work in the HepG2 cells. Thus, we proposed that one of the mechanisms of synergism between AZT and As2O3 might be through a modulation of AQP9 expression.
Methods
Chemicals and reagents
AZT and 3-(4,5-dimethyl thiazol-2-y)-2,5-diphenyl tetrazolium bromide (MTT) were purchased from Chemical Co. (St Louis, Michigan, USA). As2O3 was obtained from the Shui Kou Shan mining bureau of Hengyang industrial company, China. Other cell culture supplies were purchased from HyClone (Logan, UT, USA). The antibody to AQP9 and β-actin was purchased from ImmunoWay Biotechnology Co. (Newark, DE, USA). Peroxidase-Conjugated AffiniPure Goat anti-Rabbit IgG (H+L) was purchased from ZSGB-BIO Co. (Beijing, China). Real-time reverse transcription polymerase chain reaction (RT-PCR) related reagents were purchased from Promega (Madison, WI, USA). siRNA-AQP9 was purchased from Ambion (Austin, TX, USA). Transwell boyden chamber was purchased from Corning (Corning, NY, USA).
Cell culture
The human hepatocellular carcinoma cell lines HepG2 and MHCC97H cells were purchased from JRDUN Biotechnology Co. (Shanghai, China). The hepatocellular carcinoma cell lines HepG2 and MHCC97H were cultured in DMEM with a high glucose content containing 10% heat-inactivated fetal calf serum (FCS), 100 IU/mL penicillin/streptomycin at 37 °C, and were incubated in a 5% CO2 humidified atmosphere. Cells were harvested in a logarithmic phase for experiments.
Cell proliferation assay
The Cellular viability of the HepG2 and MHCC97H cells was assessed by the reduction process of MTT to formazan as described earlier (23). Cells were seeded into 96-well microtiter plates at a density of 1×104 cells/well with DMEM. After a period of 24 h, the culture medium in each well was replaced with a fresh medium and treated with a 2 µM As2O3 solution combined with different concentrations of AZT (0, 10, 20 µM) for an additional 48 h. Then, 10 µL of a MTT solution (5 mg/mL in PBS) was added to each well. After a 4 h incubation at 37 °C, the formazan salt crystals were dissolved in 100 µL of lysis solution (10% SDS in 0.01 M HCl). The absorbance (OD) values were measured spectrophotometrically at 490 nm using a microplate reader (Bio-RAD, Benchmark Plus, USA). Each experiment was performed in triplicate. The inhibition rate (IR) was calculated as follows:
IR (%)=1 − (Mean A490 of experiment − A490 of blank/A490 of control − A490 of blank).
Quantification of gene expression
Total RNA was extracted using TRIzol Reagent, according to the manufacturer’s protocol, and dissolved into RNase-free water. Then, 1 µg of RNA was reversely transcribed to cDNA. Afterward, AQP9 and β-actin mRNA were quantified by q-PCR using the SYBR Green Quantitative PCR kit following the manufacturer’s instructions. The primers for PCR are exhibited in Table 1. Reaction was carried out in a total volume of 20 µL for 40 cycles of 2 min at 95 °C, 15 s at 95 °C, 60 °C at 30 s and 30 s at 72 °C. The amplification reaction of AQP9 with respect to β-actin was analyzed with the comparative CT (ΔΔCT) method. Experiments were performed in triplicates.

Full table
Western blotting analysis
Cells were collected and lysed in a RIPA lysis buffer (Solarbio, Beijing, China) containing phenylmethylsulfonyl fluoride (MP Biomedicals, Santa Ana, CA, USA). The concentration was determined using the BCA protein assay kit (Solarbio, Beijing, China). 50 µg of the total protein was separated by 10% SDS-PAGE solution and blotted at the target protein and the β-actin antibody on a polyvinylidene fluoride (PVDF) membrane. The membrane was blocked with a 5% skimmed milk powder buffer for 90 min, in Tris-Buffered Saline Tween, and then was probed with an anti-AQP9 in a 1:300 diluted solution or an anti-β-actin antibody in a 1:5,000 diluted solution in the TBST. After being rinsed 3 times with a PBS, the membrane was re-probed with a HRP-linked anti-rabbit IgG in a 1:10,000 diluted TBST. Results were intuitively observed by the Immobilon Western chemiluminescent HRP Substrate (Millipore Corporation, Billerica, USA), according to the manufacturer’s protocol. Experiments were performed in triplicates.
RNA interference
HepG2 cells were electro-transfected with AQP9 siRNA according to the recommendations. After being digested by pancreatic enzyme, the cells were collected, counted and adjusted for density to 1~2×106/mL, 200 rpm 10 min. The supernatant was then removed and the cells were resuspended in a 100 µL nucleofector solution. The experiential cells were divided into the mock group (the blank control, only added nucleofector solution), the AQP9 siRNA group (transfected with AQP9 siRNA) and the NC group (transfected with NC-siRNA which were marked by GFP). After transfecting, 500 µL of DMEM (preheated) containing 10% heat-inactivated fetal calf serum was added to the cup, then removed. The samples for the 6-well microtiter plates were cultured in a 5% CO2 humidified atmosphere at 37 °C. The transfection efficiency of AQP9 siRNA was detected by q-PCR and western blotting. The primers of AQP9-siRNA are as following: sense 5'-UGACCUCAACUACAUGGUUTT-3' and antisense 5'-AACCAUGUAGUUGAGGUCATT-3'.
Wound healing assay
HepG2 cells were divided into a blank control group (HepG2 cells treated with the same volume of DMEM), HepG2 cells treated with the drug (2 µM As2O3 and 20 µM AZT) and the siRNA-AQP9 HepG2 cells treated with the drug (2 µM As2O3 and 20 µM AZT) groups. After a 12 h incubation time in 6-well plates, an artificial wound of the scratched cells was made using a 200 µL pipette tip. Then, it was rinsed with a PBS and the drugs from the corresponding group were added and cultured for 0, 24 and 48 h. The photos were captured using the microscope at a magnification of ×40. The migration distance of the wound was measured using the Image Pro Plus software. The experiment was repeated at least 3 times, and the data was normalized to the average of the control.
Transwell migration and invasion assays
At the 48 h post-transfection time, the above 3 groups of cells (5×104 cells per well) were moved into DMEM without FCS and the added drugs were then plated into the upper chambers of the transwell plates, and the medium supplemented with a 20% FCS was plated into the lower chambers. The membrane was fixed in a methanol solution, and then stained using hematoxylin after 24 h of incubation. Stained cells were visualized and counted under a light microscope (200×). The analysis of the cell invasion was consistent with that of cell migration. The cell invasion was detected using the Transwell assay with the Matrigel Invasion Chambers. Experiments were performed in triplicates.
Statistical analysis
The SPSS 19.0 software was used to perform comparative analysis of cell viability and the AQP9 expression by way of a one-way analysis of the variance. It was followed by a LSD-t-test, and all dates were presented as mean ± standard deviation (SD). The criterion of statistical difference was taken as P<0.05.
Results
Effect of As2O3 combined with different concentrations of AZT on the viability of HepG2 and MHCC97H cells
The inhibition effect of As2O3 combined with the different concentrations of AZT in human liver cancer HepG2 and MHCC97H cells were detected by MTT assay. As2O3 (2 µM) combined with AZT (20 µM) significantly inhibited the growth of HepG2 and MHCC97H cells at the 48 h mark (Figure 1) (P<0.05 or P<0.01). The results showed that As2O3 combined with AZT inhibited cell growth, and the optimum synergy inhibition concentration was 2 µM As2O3 combined with 20 µM AZT, which indicated a dose-dependent inhibition of cell growth.

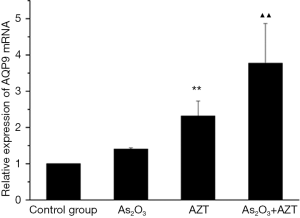
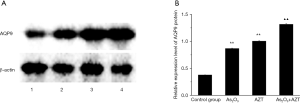
As2O3 combined with AZT up-regulated the expression of AQP9 mRNA/protein in HepG2 cells
To explain the modulating effect of As2O3 and AZT in the HepG2 cells, a real time-PCR was performed to detect the expression of the AQP9 mRNA. As shown in Figure 2, As2O3 (2 µM) and AZT (20 µM) efficiently promoted the expression of AQP9 mRNA (P<0.05). A western blotting result showed that the combination of treatments also significantly increased the protein levels of the AQP9 group (Figure 3). These results indicated that As2O3 combined with AZT could up-regulate the expressions of AQP9 mRNA/protein.
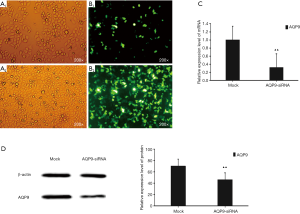
AQP9 siRNA inhibited the expression of AQP9
Since the NC-siRNA marked by GFP gene (express green fluorescent protein) is transfected into the cell, the GFP protein can be expressed in the cell and emit green fluorescence. Therefore, GFP was marked to observe the transfection efficiency. As shown in Figure 4, the green fluorescent cells were significantly increased at 10 h, so the transfection efficiency could be detected at 24 h. qPCR result showed that the transfection efficiency was nearly 70% (Figure 4C), which could meet the experimental requirements. The western blotting results showed that the protein level of AQP9 was consistent with the mRNA level (Figure 4D).
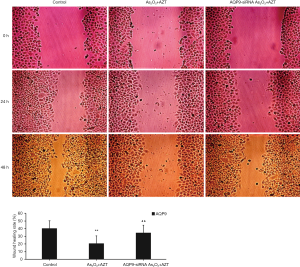
The effects of AQP9 on transversal migration of HepG2 cell
The results of the wound healing assay (Figure 5) showed that As2O3 combined with AZT could significantly inhibit the scratch healing ability of the HepG2 cell after a 24-h period. However, the effect was decreased after transfecting with AQP9 siRNA. Scratches in the blank control group basically healed 48 h after scratching. The scratch width of the AQP9-siRNA group was slightly narrower than that of the 24-h group, but there was no significant change in As2O3 when combined with the AZT group. These results indicated that As2O3 combined with AZT can significantly inhibit transversal migration of HepG2 cells, which may be controlled by AQP9.
The effects of AQP9 on longitudinal migration and invasion of HepG2 cells
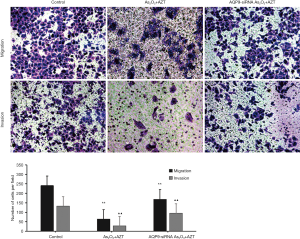
As Figure 6 illustrates, the number of transmembrane cells in the drug-treated group was significantly less than that in blank control group (P<0.01). However, the effect was reduced after transfecting with AQP9 siRNA. These results showed that As2O3 combined with AZT could significantly inhibit the longitudinal migration and the invasion of the HepG2 cells, which may be related to AQP9.
Discussion
To date, reports have shown much progress in the antineoplastic combined chemotherapy protocols of As2O3 with other drugs. As a telomerase inhibitor, AZT inhibits the infinite replication potential in the z tumor cell proliferation by inhibiting the telomerase activity (24). Also, research has shown that the combined application of the AZT with emodin can co-inhibit the cell proliferation of leukemia KG-1a, which demonstrates that by constraining the activity of telomerase, Pt-AZT suppresses Bcl-2 expression and inhibits the growth of hepatocellular carcinoma in mice (25,26). Furthermore, our previous research found that the combination of As2O3 and AZT has a remarkable therapeutic effect on the liver carcinoma cell, HepG2 (8). However, its mechanism of action has not been published in the literature.
AQP9 is widely distributed in liver tissues, which plays a significant role in the physiopathology proceedings of the liver. Li and his colleagues found that AQP9 was down-regulated in the liver carcinoma cells when compared with normal liver tissues. Meanwhile, over-expression of AQP9 inhibited the proliferation of the SMMC-7721 liver carcinoma cells (27). Other research has indicated that AQP9 promoted the intake of some micro molecule neutral solutes such as monocarboxylic acid, 5FU, platinum, and As2O3 (28). Tang and his partner reported that the over-expressed AQP9 increased the effects of As2O3 on inhibiting proliferation, migration, and the invasion of liver carcinoma cells (29). Here, we found the excitation of caspase-3 was also enhanced, which is consistent with our previous study on cell biological behavior of As2O3 combined with AZT against liver cell carcinoma HepG2. However, Gao and his colleagues reported that melanoma cells had resisted chemotherapy and radiation when there was an over-expressed AQP9 (30). These conflicting results were probably due to the effect of AQP9 being dependent on different types of cells.
In the present studies, we found that the proliferation inhibition rate of the combination of As2O3 and AZT on the hepatocellular carcinoma cells was significantly higher than As2O3 alone, and it had a dose-dependent effect for AZT. In addition, a low dose of As2O3 combined with AZT also significantly inhibited the migration and invasion of liver cell carcinoma. Furthermore, as shown by the results of qPCR, the expression levels of AQP9 in combination of As2O3 (2 µM) and AZT (20 µM) group were higher than those of As2O3 alone groups, as well as the blank control groups, which was also verified by the western blot test results.
To further investigate whether AQP9 plays a key role in the process of the combination of As2O3 and AZT against hepatocellular carcinoma cells and thus improves the treatment efficacy, the expression of AQP9 was silenced by electro-transfecting HepG2 cells with AQP9 siRNA. The results of the wound healing assay showed that the scratch width of the AQP9-siRNA group was dramatically narrower than that of As2O3 when combined with the AZT group. In addition, the number of transmembrane cells in the AQP9-siRNA group was significantly more than that of As2O3 combined with AZT group. These results suggest that AZT may increase the sensitivity of the hepatocellular carcinoma cells to As2O3 by up-regulating the expression of AQP9.
In conclusion, our study shows that As2O3 combined with AZT can co-inhibit the growth, migration and invasion of hepatocellular carcinoma cells by up-regulating the expression of AQP9. This has important implications in reducing the poisonous side effect of As2O3 in the cancer therapies.
Acknowledgments
The authors thank AME Publishing Company for English editing on this study.
Funding: This study was supported by the National Natural Science Foundation of China (No. 81460456) and the Natural Science Foundation of Gansu Province (No. 1308RJZA169).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.11.08). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Institutional ethical approval and informed consent were waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tallman MS, Altman JK. How I treat acute promyelocytic leukemia. Blood 2009;114:5126-35. [Crossref] [PubMed]
- Kojima M, Ogiya D, Ichiki A, et al. Refractory acute promyelocytic leukemia successfully treated with combination therapy of arsenic trioxide and tamibarotene: A case report. Leuk Res Rep 2016;5:11-3. [Crossref] [PubMed]
- Au WY, Kumana CR, Lee HK, et al. Oral arsenic trioxide-based maintenance regimens for first complete remission of acute promyelocytic leukemia: a 10-year follow-up study. Blood 2011;118:6535-43. [Crossref] [PubMed]
- Gu S, Chen C, Jiang X, et al. Resveratrol Synergistically Triggers Apoptotic Cell Death with Arsenic Trioxide via Oxidative Stress in Human Lung Adenocarcinoma A549 Cells. Biol Trace Elem Res 2015;163:112-23. [Crossref] [PubMed]
- Jiang L, Wang L, Chen L, et al. As2O3 induces apoptosis in human hepatocellular carcinoma HepG2 cells through a ROS-mediated mitochondrial pathway and activation of caspases. Int J Clin Exp Med 2015;8:2190-6. [PubMed]
- Wang H, Gao P, Zheng J. Arsenic trioxide inhibits cell proliferation and human papillomavirus oncogene expression in cervical cancer cells. Biochem Biophys Res Commun 2014;451:556-61. [Crossref] [PubMed]
- Chen YC, Lin-Shiau SY, Lin JK. Involvement of reactive oxygen species and Caspase 3 activation in arsenite-induced apoptosis. J Cell Physiol 1998;177:324-33. [Crossref] [PubMed]
- Chen C, Zhang YM, Wang Y, et al. Synergic effect of 3'-azido-3'- deoxythymidine and arsenic trioxide in suppressing hepatoma cells. Anti-Cancer Drugs 2011;22:435-43. [Crossref] [PubMed]
- Eyvani H, Moghaddaskho F, Kabuli M, et al. Arsenic trioxide induces cell cycle arrest and alters DNA methylation patterns of cell cycle regulatory genes in colorectal cancer cells. Life Sci 2016;167:67-77. [Crossref] [PubMed]
- Kumar S, Yedjou CG, Tchounwou PB. Arsenic trioxide induces oxidative stress, DNA damage, and mitochondrial pathway of apoptosis in human leukemia (HL-60) cells. J Exp Clin Cancer Res 2014;33:42. [Crossref] [PubMed]
- Jomova K, Jenisova Z, Feszterova M, et al. Arsenic: toxicity, oxidative stress and human disease. J Appl Toxicol 2011;31:95-107. [PubMed]
- Zhao P, Guo Y, Zhang W, et al. Neurotoxicity induced by arsenic in Gallus Gallus: Regulation of oxidative stress and heat shock protein response. Chemosphere 2017;166:238-45. [Crossref] [PubMed]
- Chiou TJ, Chu ST, Tzeng WF, et al. Arsenic trioxide impairs spermatogenesis via reducing gene expression levels in testosterone synthesis pathway. Chem Res Toxicol 2008;21:1562-9. [Crossref] [PubMed]
- Kchour G, Tarhini M, Kooshyar MM, et al. Phase 2 study of the efficacy and safety of the combination of arsenic trioxide, interferon alpha, and zidovudine in newly diagnosed chronic adult T-cell leukemia/lymphoma (ATL). Blood 2009;113:6528-32. [Crossref] [PubMed]
- Namba T, Kodama R, Moritomo S, et al. Zidovudine, an anti-viral drug, resensitizes gemcitabine-resistant pancreatic cancer cells to gemcitabine by inhibition of the Akt-GSK3β-Snail pathway. Cell Death Dis 2015;6:e1795 [Crossref] [PubMed]
- Liu Y, Yuan LY, Chu HY, et al. As2O3 in combination with AZT suppresses the proliferation of hepatoma HepG2 cells through activating caspase-3 pathway. Tumor 2014;34:705-11.
- Han L, Chen C, Chu HY. Effects of As2O3 combined with AZT on migration and invasion of liver cancer HepG2 cells. Tumor 2015;35:982-9.
- Rehman K, Naranmandura H. Double-Edged Effects of Arsenic Compounds: Anticancer and Carcinogenic Effects. Current Drug Metabolism 2013;14:1029-41. [Crossref] [PubMed]
- Bhattacharjee H, Carbrey J, Rosen BP, et al. Drug uptake and pharmacological modulation of drug sensitivity in leukemia by AQP9. Biochem Biophys Res Commun 2004;322:836-41. [Crossref] [PubMed]
- Leung J, Pang A, Yuen WH, et al. Relationship of expression of aquaglyceroporin 9 with arsenic uptake and sensitivity in leukemia cells. Blood 2007;109:740-6. [Crossref] [PubMed]
- Chau D, Ng K, Chan TSY, et al. Azacytidine sensitizes acute myeloid leukemia cells to arsenic trioxide by up-regulating the arsenic transporter aquaglyceroporin 9. J Hematol Oncol 2015;8:46. [Crossref] [PubMed]
- Chen Q, Zhu L, Zheng B, et al. Effect of AQP9 Expression in Androgen-Independent Prostate Cancer Cell PC3. Int J Mol Sci 2016;17:E738 [Crossref] [PubMed]
- Alley MC, Scudiero DA, Monks A, et al. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res 1988;48:589-601. [PubMed]
- Armando RG, Gomez DM, Gomez DE. AZT exerts its antitumoral effect by telomeric and non-telomeric effects in a mammary adenocarcinoma model. Oncol Rep 2016;36:2731-6. [Crossref] [PubMed]
- Wang LN, Li ZJ, Xi YM, et al. Effect of Emodin Combined with AZT on the Proliferation and the Expression of BCL-2, NF-κB, TGF-β in the Leukemia Stem Cells-KG-1a cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2015;23:1265-71. [PubMed]
- Sabokrouh A, Vaisiraygani A, Goodarzi MT, et al. Comparison between Platinum-Azidothymidine and Azidothymidine Effects on Bcl-2 and Telomerase Gene Expression in Rats with Hepatocellular Carcinoma. Avicenna J Med Biotechnol 2015;7:50-6. [PubMed]
- Li CF, Zhang WG, Liu M, et al. Aquaporin 9 inhibits hepatocellular carcinoma through up-regulating FOXO1 expression. Oncotarget 2016;7:44161-70. [PubMed]
- Wang Y, Yin JY, Li XP, et al. The association of transporter genes polymorphisms and lung cancer chemotherapy response. PLoS One 2014;9:e91967 [Crossref] [PubMed]
- Tang J, Wang C, Jiang Z. The expression of AQP9 in HepG2 cells affects cell biological behaviors and sensitivity to As2O3. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2015;31:769-74. [PubMed]
- Gao L, Gao Y, Li X. Aquaporins mediate the chemoresistance of human melanoma cells to arsenite. Mol Oncol 2012;6:81-7. [Crossref] [PubMed]


