
The albumin-bilirubin score stratifies the outcomes of Child-Pugh class A patients after resection of hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) represents the sixth most common tumour and the second cause of cancer-related death worldwide (1). Surgery is a potentially curative treatment for HCC patients, specifically in early stage and with well-preserved liver function (2). The majority of patients with HCC have associated chronic liver disease (3), ranging from few histological abnormalities to advanced cirrhosis (4-6), resulting in a higher risk of postoperative complications (7,8), particularly post-hepatectomy liver failure (PHLF), which is the main cause of death after liver resection (9).
Moreover, the role of clinically significant portal hypertension (CSPH) is still controversial and initially considered as an absolute contraindication for liver resection. However recent surgical series have reported good results for patients submitted to limited hepatectomies (10-12).
The Child-Pugh (CP) score is a widely used tool to assess liver function that can estimate postoperative outcomes in patients with HCC (13); however, CP score has many limitations (14). For instance, the majority of patients who undergo surgery for HCC belongs to CP class A (15), even if a wide variation in the degree of hepatic reserve was demonstrated among this group of patients (16-18). In addition, some of the variables considered in the CP score can be highly subjective. In order to overcome its limitations, additional quantitative measures of liver function have been proposed.
Recently, Johnson et al. (19) proposed the albumin-bilirubin (ALBI) score as a new score for assessing liver function in patients with HCC, demonstrating its superiority over CP in predicting overall survival (OS) in these patients.
The ALBI score is based on a simple mathematical formula involving only two variables, serum albumin and bilirubin levels, and stratifies patients with HCC into three liver function risk categories. Furthermore, the authors showed that this model can distinguish two different prognostic categories among CP class A patients.
Therefore, we sought to provide evidence that the ALBI score can identify distinct prognostic groups among CP class A patients undergoing liver resection with curative intent and to verify the ability of the ALBI score to predict short-term and long-term outcomes. An additional aim was to verify if the ALBI score could stratify long-term outcomes for patients with CSPH or for patients with different BCLC stages.
Methods
From January 2006 to December 2016, 187 CP class A patients who underwent liver resections with curative intent for HCC in the Surgical Division of the Department of Surgery at the University of Verona were included in the study.
The diagnosis of HCC was made according to the American Association for the Study of Liver Diseases (AASLD) criteria (20).
All patients were tested for HBV and HCV infection. Liver function tests included bilirubin, AST, ALT, GGT, albumin, prothrombin time, creatinine and sodium serum levels. CSPH was evaluated according to the AASLD Guidelines (21,22) by the assessment of esophageal varices or by the combination of platelet count <150,000/mm3 and splenomegaly on imaging.
Surgical resection was the treatment of choice for patients with single HCC in the absence of CSPH. Surgical resection was performed in selected cases with oligofocal HCC or in the presence of CSPH.
The extent of resection was defined according to the classification of Brisbane (23).
The ALBI score was calculated by the following formula: 0.66 × log10[total bilirubin (µmol/L)] – 0.085[albumin (g/L)] (19). The ALBI score was stratified as grade 1 (−2.60 or less), grade 2 (−2.59 to −1.39), or grade 3 (greater than −1.39).
Pathologic findings a single pathologist expert in liver disease (P Capelli) and included the tumour characteristics, tumour grade, vascular invasion, microsatellite lesions, and width of the surgical margins. The surgical resection margin was defined as the shortest distance from the edge of the tumour to the line of transection, as detected by histological examination of the resected specimen.
The underlying liver disease and staging of cirrhosis was classified according to the scoring system from Ishak et al. (24). The steatosis grade, lobular inflammation, hepatocyte ballooning and extent of fibrosis were reported as described by Kleiner et al. (25).
Short-term endpoints included postoperative mortality and morbidity, which were defined as events occurring during the hospital stay or within 90 days after surgery. Postoperative morbidity was classified according to the Dindo-Clavien classification (26).
PHLF was defined as failure of one or more synthetic and excretory functions, leading to hyperbilirubinemia, hypoalbuminemia, prolonged prothrombin time, elevated serum lactate and different grades of hepatic encephalopathy (27,28). PHLF was classified according to definition from the International Study Group of Liver Surgery (ISGLS) (29). Postoperative ascites was defined as drainage output greater than 500 cc/day or significant weight gain after surgery and confirmed ascites with ultrasound or CT.
After liver resection, patients were monitored every 3 months by physical examination and serum AFP levels, and imaging follow-up was performed every 6 months with an abdominal ultrasound and/or a CT or an MRI.
Long-term outcome included survival, which was defined as death after surgery for cancer or end-stage liver disease. The study was reviewed and approved by the Ethics Committee of University of Verona, Verona, Italy, with ID number: 102 CESC.
A written consent for data collection and processing was provided by all study participants.
Statistical analysis
Data were collected and analysed with SPSS statistical software (SPSS version 21.0 Inc., Chicago, IL, USA). The differences between categorical variables were analysed with the χ2 test. The differences between the means of continuous variables were analysed with Student’s t-test. Survival analysis was carried out with the Kaplan-Meier method. Univariate analysis for survival was performed with the Kaplan-Meier method, and the log rank test was used to verify the significance of differences. A multivariate analysis for survival was performed with Cox’s regression model, and significant variables from the univariate analysis were included. A P value lower than 0.05 was considered statistically significant.
Results
The characteristics of 187 patients CP class A are shown in Table 1. No patients were classified as ALBI 3, 125 (66.8%) were ALBI 1, and 62 (33.2%) were ALBI 2.
Table 1
| Characteristic | All patients, 187 patients, n (%) | ALBI 1, 125 patients, n (%) | ALBI 2, 62 patients, n (%) | P value |
|---|---|---|---|---|
| Gender | 0.770 | |||
| Male | 153 (81.8) | 103 (82.4) | 50 (80.6) | |
| Female | 34 (18.2) | 22 (17.6) | 12 (19.4) | |
| Mean age [range] | 67.7 [34–93] | 68.0 [34–87] | 67.2 [34–93] | 0.628 |
| Aetiology | 0.006 | |||
| MS | 38 (20.3) | 32 (25.6) | 6 (9.7) | |
| Alcohol | 37 (19.8) | 26 (20.8) | 11 (17.7) | |
| Virus | 86 (46.0) | 47 (37.6) | 39 (62.9) | |
| Cryptogenic | 26 (13.9) | 20 (16.0) | 6 (9.7) | |
| BCLC | 0.702 | |||
| A | 95 (50.8) | 66 (52.8) | 29 (46.8) | |
| B | 63 (33.7) | 41 (32.8) | 22 (35.5) | |
| C | 29 (15.5) | 18 (14.4) | 11 (17.7) | |
| Platelets | 0.021 | |||
| ≤150,000 | 56 (29.9) | 30 (24.0) | 25 (40.3) | |
| >150,000 | 131 (70.1) | 95 (76.0) | 37 (59.7) | |
| Esophageal varices | 0.093 | |||
| Yes | 19 (10.2) | 9 (7.2) | 9 (14.5) | |
| No | 168 (89.8) | 116 (92.8) | 53 (85.5) | |
| CSPH | 0.053 | |||
| Yes | 60 (32.1) | 34 (27.2) | 26 (41.9) | |
| No | 127 (67.9) | 91 (72.8) | 36 (58.1) | |
| Major hepatectomy | 0.212 | |||
| Yes | 44 (23.5) | 26 (20.8) | 18 (29.0) | |
| No | 143 (76.5) | 99 (79.2) | 44 (71.0) | |
| AFP (ng/mL) | 0.168 | |||
| ≥200 | 41 (21.9) | 23 (18.4) | 17 (27.4) | |
| <200 | 146 (78.1) | 102 (81.6) | 45 (72.6) | |
| Fibrosis | 0.001 | |||
| 4 | 98 (52.4) | 54 (43.2) | 44 (71.0) | |
| 1–3 | 89 (47.6) | 71 (56.8) | 18 (29.0) | |
| No. of nodes | 0.895 | |||
| Single | 159 (85.0) | 107 (85.6) | 53 (85.5) | |
| Multiple | 28 (15.0) | 18 (14.4) | 9 (14.5) | |
| Peri-tumoral capsule | 0.755 | |||
| Yes | 124 (66.3) | 82 (65.6) | 42 (67.7) | |
| No | 63 (33.7) | 43 (34.4) | 20 (32.3) | |
| Microvascular invasion | 0.106 | |||
| Yes | 110 (58.8) | 69 (55.2) | 43 (69.4) | |
| No | 77 (41.2) | 56 (44.8) | 19 (30.6) | |
| Tumour size, >50 mm | 0.195 | |||
| Yes | 70 (37.4) | 43 (34.4) | 27 (43.5) | |
| No | 117 (62.6) | 82 (65.6) | 35 (46.5) |
CP, Child-Pugh; HCC, hepatocellular carcinoma; ALBI, albumin-bilirubin; CSPH, clinically significant portal hypertension.
ALBI 2 patients had more frequently a viral aetiology (P=0.006), splenomegaly (P=0.033), CSPH (P=0.053), stage 4 fibrosis (P<0.001) and lower platelet counts (P=0.021).
CSPH was identified in 60 (32.1%) patients; their characteristics are shown in Table 2. Patients with CSPH more frequently had a viral aetiology (63.3%, P<0.001) and stage 4 fibrosis (88.3%, P<0.001) and underwent minor hepatectomy (88.3%, P=0.013).
Table 2
| Characteristic | CSPH no, 127 patients, n (%) | CSPH yes, 60 patients, n (%) | P value |
|---|---|---|---|
| ALBI | 0.053 | ||
| 1 | 91 (71.7) | 34 (56.7) | |
| 2 | 36 (28.3) | 26 (43.3) | |
| Gender | 0.521 | ||
| Female | 22 (17.3) | 13 (21.7) | |
| Male | 105 (82.7) | 47 (78.3) | |
| Aetiology | <0.001 | ||
| MS | 33 (26.0) | 5 (8.3) | |
| Alcohol | 23 (18.1) | 14 (23.3) | |
| Virus | 47 (37.0) | 38 (63.3) | |
| Cryptogenic | 24 (18.9) | 3 (5.0) | |
| AST (U/L) | 0.033 | ||
| <70 | 95 (74.8) | 32 (53.3) | |
| ≥70 | 32 (25.2) | 28 (46.7) | |
| ALT (U/L) | 0.011 | ||
| <70 | 100 (78.7) | 36 (60.0) | |
| ≥70 | 27 (21.3) | 24 (40.0) | |
| Bilirubin (mg/dL) | 0.139 | ||
| <2 | 124 (97.6) | 56 (93.3) | |
| ≥2 | 3 (2.4) | 4 (6.7) | |
| AFP (ng/mL) | 0.251 | ||
| <200 | 103 (81.1) | 44 (73.3) | |
| ≥200 | 24 (18.9) | 16 (26.7) | |
| Fibrosis | <0.001 | ||
| 1–3 | 82 (64.6) | 7 (11.7) | |
| 4 | 45 (35.4) | 53 (88.3) | |
| No. of nodes | 0.935 | ||
| Single | 108 (85.0) | 51 (85.0) | |
| Multiple | 19 (15.0) | 9 (15.0) | |
| Tumour size (mm) | 0.062 | ||
| <50 | 75 (59.1) | 44 (73.3) | |
| ≥50 | 52 (40.9) | 16 (26.7) | |
| BCLC | 0.450 | ||
| A | 62 (48.8) | 35 (58.3) | |
| B | 46 (36.2) | 16 (26.7) | |
| C | 19 (15.0) | 9 (15.0) | |
| Major hepatectomy | 0.013 | ||
| No | 90 (70.9) | 53 (88.3) | |
| Yes | 37 (29.1) | 7 (11.7) |
HCC, hepatocellular carcinoma; CSPH, clinically significant portal hypertension; ALBI, albumin-bilirubin.
Patient characteristics among the different BCLC stages showed that ALBI score was similar among different groups. Specifically, 67 (70.5%), 41 (65.1%) and 18 (62.1%) ALBI 1 patients were classified in the BCLC A, BCLC B and BCLC C stage groups, respectively (P=0.702) (Table 3). In addition, aetiology, bilirubin level and severity of fibrosis were equally distributed among the BCLC groups (Table 3). As expected, BCLC B stage and BCLC C stage groups showed more advanced liver tumours with higher AFP values, more multinodular tumours and larger tumours (Table 3).
Table 3
| Characteristic | BCLC A patients, n (%) (n=95) | BCLC B patients, n (%) (n=63) | BCLC C patients, n (%) (n=29) | P value |
|---|---|---|---|---|
| ALBI | 0.702 | |||
| 1 | 67 (70.5) | 41 (65.1) | 18 (62.1) | |
| 2 | 28 (29.5) | 22 (34.9) | 11 (37.9) | |
| Gender | 0.243 | |||
| Female | 17 (17.9) | 14 (22.2) | 3 (10.3) | |
| Male | 78 (82.1) | 49 (77.8) | 26 (89.7) | |
| Aetiology | 0.909 | |||
| MS | 18 (18.9) | 14 (22.2) | 6 (20.7) | |
| Alcohol | 19 (20.0) | 11 (17.5) | 7 (24.1) | |
| Virus | 45 (47.4) | 26 (41.3) | 15 (51.7) | |
| Cryptogenic | 13 (13.7) | 12 (19.0) | 1 (3.4) | |
| AST (U/L) | 0.685 | |||
| <70 | 67 (70.5) | 43 (68.3) | 19 (65.5) | |
| ≥70 | 28 (29.5) | 20 (31.7) | 10 (34.5) | |
| ALT (U/L) | 0.011 | |||
| <70 | 72 (75.8) | 49 (77.8) | 17 (58.6) | |
| ≥70 | 23 (24.2) | 14 (22.2) | 12 (41.4) | |
| Bilirubin (mg/dL) | 0.479 | |||
| <2 | 92 (96.8) | 61 (96.8) | 29 (100) | |
| ≥2 | 3 (3.2) | 2 (3.2) | 0 (0) | |
| AFP (ng/mL) | 0.046 | |||
| <200 | 82 (86.3) | 46 (73.0) | 18 (62.1) | |
| ≥200 | 13 (13.7) | 17 (27.0) | 11 (37.9) | |
| Fibrosis | 0.415 | |||
| 1–3 | 41 (43.2) | 34 (54.0) | 13 (44.8) | |
| 4 | 54 (56.8) | 29 (46.0) | 16 (55.2) | |
| No. of nodes | 0.004 | |||
| Single | 87 (91.6) | 53 (84.1) | 19 (65.5) | |
| Multiple | 8 (8.4) | 10 (15.9) | 10 (34.5) | |
| Tumour size (mm) | 0.001 | |||
| <50 | 93 (97.9) | 7 (11.1) | 17 (58.6) | |
| ≥50 | 2 (2.1) | 56 (88.9) | 12 (41.4) | |
| Major hepatectomy | 0.001 | |||
| No | 91 (95.8) | 39 (61.9) | 13 (44.8) | |
| Yes | 4 (4.2) | 24 (38.1) | 16 (55.2) |
ALBI, albumin-bilirubin.
Short-term outcomes
The postoperative complication rates are shown in Table 4. The overall complication rate did not differ between the ALBI groups, with a frequency of 46.4% and 48.4% for ALBI 1 and ALBI 2, respectively (P=0.247). The frequency of surgical complications, such as biliary leakage, collection, sepsis and bleeding, was not significantly different among the different groups. The frequency of ascites was higher in ALBI 2 patients (16, 25.8%) than in ALBI 1 patients (17, 13.6%) (P=0.016). According to the ISGLS definition, grade A was observed in all 9 patients who developed PHLF, and the incidence of PHLF was 3 (2.4%) and 6 (9.7%) for ALBI 1 and ALBI 2, respectively (P=0.027).
Table 4
| Variable | All patients, 187 patients, n (%) | ALBI 1, 125 patients, n (%) | ALBI 2, 62 patients, n (%) | P value |
|---|---|---|---|---|
| Complications | 88 (47.1) | 58 (46.4) | 30 (48.4) | 0.247 |
| PHLF | 0.027 | |||
| A | 9 (4.8) | 3 (2.4) | 6 (9.7) | |
| B | 0 (0) | 0 (0) | 0 (0) | |
| C | 0 (0) | 0 (0) | 0 (0) | |
| Ascites | 33 (17.6) | 17 (13.6) | 16 (25.8) | 0.016 |
| Sepsis | 4 (2.1) | 3 (2.4) | 1 (1.6) | 0.632 |
| Bile leak | 11 (5.9) | 8 (6.4) | 3 (4.8) | 0.536 |
| Intra-abdominal abscess | 12 (6.4) | 8 (6.4) | 4 (6.5) | 0.545 |
| Postoperative mortality | 4 (2.1) | 3 (2.4) | 1 (1.6) | 0.632 |
HCC, hepatocellular carcinoma; ALBI, albumin-bilirubin; PHLF, post-hepatectomy liver failure.
Postoperative mortality was not significantly different among the two groups at 2.4% and 1.6% for ALBI 1 and ALBI 2, respectively (P=0.632). In the ALBI 1 group, the cause of death was sepsis in one patient who underwent combined liver and colon resection and a cardiac arrhythmia in two patients, and in the ALBI 2 group, the cause of single death was myocardial infarction.
Long-term outcomes
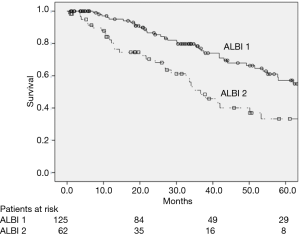
The 5-year OS was 49.2% in the entire cohort and OS was significantly longer in ALBI 1 patients than in ALBI 2 patients, with a 5-years OS of 57.1% and 33.5% for ALBI 1 and ALBI 2, respectively (P=0.001) (Figure 1).
According to BCLC stage, OS was 56.0%, 51.1% and 22.5% in BCLC A, BCLC B, and BCLC C stage patients, respectively (P<0.001).
Factors related with survival in the univariate analysis were as follows: AFP levels ≥200 ng/mL (P=0.008), multiple nodules (P=0.003), the presence of stage 4 fibrosis (P=0.033) and vascular invasion (P=0.001) .
The multivariate analysis with Cox’s regression model confirmed that the ALBI score [hazard ratio (HR) 1.9, 95% CI: 1.0–3.5, P=0.026], stage 4 fibrosis (HR 2.0, 95% CI: 1.1–3.8, P=0.020) and the presence of microvascular invasion (HR 3.1, 95% CI: 1.6–5.9, P=0.001) were factors related to survival (Table 5).
Table 5
| Variable | Median survival months (95% CI) | Univariate | Multivariate | |||
|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | |||
| Gender | ||||||
| Male | 57.9 (16.2–83.7) | Ref | 0.171 | |||
| Female | 50.1 (16.6–83.7) | 1.4 (0.8–2.5) | ||||
| Aetiology | ||||||
| MS | Not reached | Ref | ||||
| Alcohol | 53.2 (35.1–71.3) | 1.8 (0.7–4.3) | 0.152 | |||
| Virus | 50.1 (36.1–64.1) | 1.9 (0.8–4.0) | 0.095 | |||
| Other | 57.9 (18.4–97.4) | 1.6 (0.6–4.5) | 0.291 | |||
| BCLC | ||||||
| A | 63.8 (46.0–81.6) | Ref | – | NS | ||
| B | 66.9 (–) | 1.2 (0.6–2.1) | 0.496 | |||
| C | 26.6 (17.9–35.3) | 2.7 (1.4–4.9) | 0.001 | |||
| Platelets | ||||||
| ≤150,000 | 73.7 (50.6–96.8) | Ref | – | NS | ||
| >150,000 | 41.9 (34.2–49.6) | 1.5 (0.9–2.5) | 0.073 | |||
| CSPH | ||||||
| No | 73.7 (50.6–96.8) | Ref | ||||
| Yes | 41.6 (26.6–57.3) | 1.4 (0.9–2.4) | 0.102 | |||
| ALBI score | ||||||
| 1 | 73.6 (54.6–92.8) | Ref | 0.001 | Ref | 0.026 | |
| 2 | 36.6 (30.3–43.0) | 2.0 (1.2–3.2) | 1.9 (1.0–3.5) | |||
| AFP (ng/mL) | ||||||
| <200 | 65.8 (43.5–84.0) | Ref | 0.008 | – | ||
| >200 | 35.2 (19.5–50.8) | 1.6 (0.9–2.8) | ||||
| Fibrosis | ||||||
| 1–3 | 74.2 (–) | Ref | Ref | 0.020 | ||
| 4 | 41.9 (27.5–56.4) | 1.7 (1.0–2.7) | 0.033 | 2.0 (1.1–3.8) | ||
| Number | ||||||
| Single | 63.8 (46.7–80.9) | Ref | – | NS | ||
| Multiple | 32.6 (23.3–42.0) | 2.4 (1.3–4.4) | 0.003 | |||
| Microvascular invasion | ||||||
| No | 102.0 (57.2–146.7) | Ref | Ref | |||
| Yes | 36.8 (25.1–48.4) | 2.8 (1.5–4.7) | 0.001 | 3.1 (1.6–5.9) | 0.001 | |
| Tumour size (mm) | ||||||
| <50 | 57.9 (42.0–73.8) | Ref | 0.147 | |||
| >50 | 49.2 (19.0–79.4) | 1.4 (0.8–2.2) | ||||
OS, overall survival; HCC, hepatocellular carcinoma; CSPH, clinically significant portal hypertension, NS, not significant.
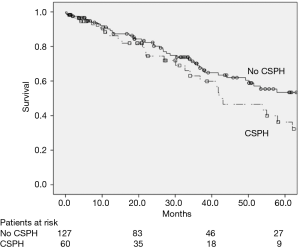
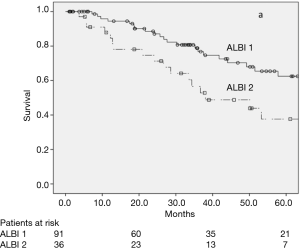
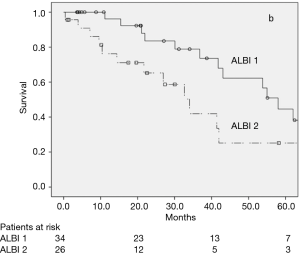
CSPH was not related to OS in the univariate analysis (Figure 2), with a 5-year OS rate of 54.8% and 37.0% (P=0.102) for patients with or without CSPH, respectively. In the subgroup analysis of patients without CSPH, the 5-year OS rates were 62.5% and 37.6% for ALBI 1 and ALBI 2, respectively (P=0.021) (Figure 3), and in patients with CSPH the 5-year OS rates were 44.6% and 25.2% for ALBI 1 and ALBI 2, respectively (P=0.031) (Figure 4).
Further OS subgroup analysis among the different BCLC stages demonstrated longer OS in BCLC A patients, with OS of 56.0%, 51.1% and 22.5% in BCLC A, BCLC B, and BCLC C stage patients, respectively (P<0.001). The ALBI score was also able to further classify OS among the different BCLC stage groups. In particular, the 5-year OS in BCLC A stage patients was 58.9% and 36.5% for ALBI 1 and ALBI 2, respectively (P=0.092), in BCLC B stage patients was 56.9% and 46.3% for ALBI 1 and ALBI 2, respectively (P=0.259), and in BCLC C stage patients was 43.7% and 0% for ALBI 1 and ALBI 2, respectively (P=0.017).
Discussion
Tumour stage and underlying liver function influences the prognosis of patients with HCC who undergo surgery with curative intent. Surgery is usually indicated for CP class A HCC patients without CSPH. Although several limitations of CP score, it remains a widely used tool for estimating preoperative liver function.
The ALBI score is a new model for assessing liver functional reserve in patients with HCC. It is easy to calculate since it includes two commonly measured variables and it can be represented using a simple nomogram. Another strong element that supports the use of this classification is that the variables are evaluated objectively.
The ALBI score was introduced by Johnson et al. (19) and validated in a surgical series (30). These authors proved that this model is better than CP score at predicting the OS of patients who undergo surgery for HCC.
In the present study, we found that the ALBI score had discriminatory role in CP class A HCC patients who have undergone resection for predicting short- and long-term outcomes.
Similarly, Wang described that the incidence of PHLF is related to the ALBI score (1, 2 and 3) in both major (25 of 175, 35 of 91 and 1 of 1; P<0.001) and minor (44 of 675, 60 of 299 and 1 of 1; P<0.001) resections. Moreover, ALBI 2 patients had increased risk for postoperative complications, such as ascites and PHLF.
In the literature, a non-surgical series reported similar results. Johnson et al. (19) also described the discriminatory power of ALBI score in CP class A patients, and they observed a 10-month difference in survival between the two ALBI grades independent of treatment approach. Gui et al. (31) found that the ALBI score revealed two classes with different prognoses for patients treated with radioembolization. Patients with ALBI grade 1 had improved survival over patients with ALBI grade 2 (P=0.01), with a median survival time of 16.7 months (95% CI, 12.3–56.9 months) compared with a median survival of 9.8 months (95% CI, 7.3–13.2 months) in patients with ALBI grade 2.
A surgical series by Wang et al. (30) described that their study population OS did not differ between patients with CP class A and class B (P=0.055). However, the OS at 1, 3 and 5 years was higher in patients with ALBI grade 1 (84%, 68% and 59.4%) than in those with ALBI grade 2 (76.4%, 52.5% and 38%; P<0.001).
Another aim of our study was to evaluate the ability of the ALBI score to predict the prognosis among patients with CSPH. CSPH can affect morbidity and mortality in patients undergoing liver resection for HCC. Surgery is usually recommended for HCC patients without CSPH; however, the presence of CSPH is not an absolute contraindication for liver resection (32), even if it can influence the prognosis in terms of morbidity and mortality (32-34). We found that the ALBI score can stratify OS among patients with CSPH, and there are no other papers that have described this correlation.
The OS was not significantly different between patients with or without CSPH; however, by applying the ALBI score to these two groups, we found that the ALBI score could stratify the OS in patients with or without CSPH. This finding could be useful to better select patients with portal hypertension for undergoing surgery with curative intent.
We further applied the ALBI score among the different BCLC stages and found that OS was longer in ALBI 1 patients in BCLC A, B and C stages. These findings confirm that the ALBI score can improve patient selection compared to the current staging system and may have clinical applications for the preoperative selection of treatment.
Similar findings were reported in other studies in the literature. Ma et al. (35) tested the ALBI score among patients with early-stage HCC (i.e., with BCLC stage 0 and A) and found that the OS of early-stage patients with ALBI grade 1 was longer than those with ALBI grade 2, and early-stage HCC patients with ALBI grade 2 experienced significantly shorter OS (P<0.001) and higher death rates. In a large multicentre cohort of 3,696 surgical and non-surgical cases of HCC, Chan et al. (36) demonstrated that the ALBI score stratified OS among all the BCLC stages. A western study (37) involving 2,426 HCC patients defined the ALBI score as a significant predictor of patient OS after surgical resection (P<0.001), transarterial chemoembolization (P<0.001) and sorafenib treatment (P<0.001), and stratification by the Barcelona Clinic Liver Cancer system confirmed the independent prognostic value of the ALBI score across diverse stages of the disease.
Several limitations should be considered when interpreting the present study. No ALBI 3 patients were found in our study population. Another limitation is the retrospective nature of the study, and the statistical analysis results may be suboptimal due to the relatively small sample size and low mortality rate. Thus, our results should be confirmed and validated with larger series by other institutions.
Finally, a limitation of the ALBI score is that it includes only two quantitative variables.
Conclusions
The ALBI score is a new prognostic score in patients with HCC and should be considered before hepatic resection. In this study, we underline how the ALBI score represents a simple tool to better assess liver function and stratify the risk of postoperative complications in CP class A HCC patients undergoing surgery. Moreover, our data suggest that the ALBI score seems to efficiently predict the long-term survival among patients with CSPH.
This study supports the use of the ALBI score in clinical practice to better select patients before surgery.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Giovanni Brandi; Francesco Tovoli) for the series “Primary Liver Cancer” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.12.10). The series “Primary Liver Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of University of Verona, Verona, Italy (No. 102 CESC) and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Vitale A, Burra P, Frigo AC, et al. Survival benefit of liver resection for patients with hepatocellular carcinoma across different Barcelona Clinic Liver Cancer stages: a multicenter study. J Hepatol 2015;62:617-24. [Crossref] [PubMed]
- Zhong JH, Ke Y, Gong WF, et al. Hepatic resection associated with good survival for selected patients with intermediate and advanced-stage hepatocellular carcinoma. Ann Surg 2014;260:329-40. [Crossref] [PubMed]
- Guo Z, Zhong JH, Jiang JH, et al. Comparison of survival of patients with BCLC stage A hepatocellular carcinoma after hepatic resection or transarterial chemoembolization: a propensity score-based analysis. Ann Surg Oncol 2014;21:3069-76. [Crossref] [PubMed]
- El-Serag HB. Hepatocellularcarcinoma. N Engl J Med 2011;365:1118-27. [Crossref] [PubMed]
- Johnson PJ, Williams R. Cirrhosis and the etiology of hepatocellular carcinoma. J Hepatol 1987;4:140-7. [Crossref] [PubMed]
- Farges O, Malassagne B, Flejou JF, et al. Risk of major liver resection in patients with underlying chronic liver disease: a reappraisal. Ann Surg 1999;229:210-5. [Crossref] [PubMed]
- McCormack L, Petrowsky H, Jochum W, et al. Hepatic steatosis is a risk factor for postoperative complications after major hepatectomy: a matched case-control study. Ann Surg 2007;245:923-30. [Crossref] [PubMed]
- Schreckenbach T, Liese J, Bechstein WO, et al. Posthepatectomy liver failure. Dig Surg 2012;29:79-85. [Crossref] [PubMed]
- Guglielmi A, Ruzzenente A, Conci S, et al. Hepatocellular carcinoma: surgical perspectives beyond the barcelona clinic liver cancer recommendations. World J Gastroenterol 2014;20:7525-33. [Crossref] [PubMed]
- Cucchetti A, Ercolani G, Vivarelli M, et al. Is portal hypertension a contraindication to hepatic resection? Ann Surg 2009;250:922-8. [Crossref] [PubMed]
- Santambrogio R, Kluger MD, Costa M, et al. Hepatic resection for hepatocellular carcinoma in patients with Child- Pugh’s A cirrhosis: is clinical evidence of portal hypertension a contraindication? HPB (Oxford) 2013;15:78-84. [Crossref] [PubMed]
- European Association For The Study Of The Liver. EASL-EORTC clinical practice guidelines:management of hepatocellular carcinoma. J Hepatol 2012;56:908-43. [Crossref] [PubMed]
- Durand F, Valla D. Assessment of the prognosis of cirrhosis: Child-Pugh versus MELD. J Hepatol 2005;42:S100-7. [Crossref] [PubMed]
- Johnson P, Berhane S, Kagebayashi C, et al. Impact of disease stage and aetiology on survival in hepatocellular carcinoma: implications for surveillance. Br J Cancer 2017;116:441-7. [Crossref] [PubMed]
- Schneider PD. Preoperative assessment of liver function. Surg Clin North Am 2004;84:355-73. [Crossref] [PubMed]
- Seyama Y, Kokudo N. Assessment of liver function for safe hepatic resection. Hepatol Res 2009;39:107-16. [Crossref] [PubMed]
- Fan ST. Liver functional reserve estimation: state of the art and relevance for local treatments:the Eastern perspective. J Hepatobiliary Pancreat Sci 2010;17:380-4. [Crossref] [PubMed]
- Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach - the ALBI grade. J Clin Oncol 2015;33:550-8. [Crossref] [PubMed]
- Bruix J, Sherman MAmerican Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020-2. [Crossref] [PubMed]
- Garcia-Tsao G, Abraldes JG, Berzigotti A, et al. Portal Hypertensive Bleeding in Cirrhosis: Risk Stratification, Diagnosis, and Management: 2016 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2017;65:310-35. [Crossref] [PubMed]
- Augustin S, Millán L, González A, et al. Detection of early portal hypertension with routine data and liver stiffness in patients with asymptomatic liver disease: a prospective study. J Hepatol 2014;60:561-9. [Crossref] [PubMed]
- Belghiti J, Clavien P, Gadzijev E, et al. The Brisbane 2000 terminology of liver anatomy and resections. HPB 2000;2:333-9. [Crossref]
- Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol 1995;22:696-9. [Crossref] [PubMed]
- Kleiner DE, Brunt EM, Van Natta M, et al. Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313-21. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- van den Broek MA, Olde Damink SW, Dejong CH, et al. Liver failure after partial hepatic resection:definition, pathophysiology, risk factors and treatment. Liver Int 2008;28:767-80. [Crossref] [PubMed]
- Imamura H, Seyama Y, Kokudo N, et al. One thousand fifty-six hepatectomies without mortality in 8 years. Arch Surg 2003;138:1198-206; discussion 1206. [Crossref] [PubMed]
- Balzan S, Belghiti J, Farges O, et al. The ‘50- 50 criteria’ on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg 2005;242:824-8; discussion 828-9. [Crossref] [PubMed]
- Wang YY, Zhong JH, Su ZY, et al. Albumin-bilirubin versus Child-Pugh score as a predictor of outcome after liver resection for hepatocellular carcinoma. Br J Surg 2016;103:725-34. [Crossref] [PubMed]
- Gui B, Weiner AA, Nosher J, et al. Assessment of the Albumin-Bilirubin (ALBI) Grade as a Prognostic Indicator for Hepatocellular Carcinoma Patients Treated With Radioembolization. Am J Clin Oncol 2018;41:861-6. [Crossref] [PubMed]
- Cucchetti A, Cescon M, Golfieri R, et al. Hepatic venous pressure gradient in the preoperative assessment of patients with resectable hepatocellular carcinoma. J Hepatol 2016;64:79-86. [Crossref] [PubMed]
- Ruzzenente A, Valdegamberi A, Campagnaro T, et al. Hepatocellular carcinoma in cirrhotic patients with portal hypertension: is liver resection always contraindicated? World J Gastroenterol 2011;17:5083-8. [Crossref] [PubMed]
- Ishizawa T, Hasegawa K, Aoki T, et al. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology 2008;134:1908-16. [Crossref] [PubMed]
- Ma XL, Zhou JY, Gao XH, et al. Application of the albumin-bilirubin grade for predicting prognosis after curative resection of patients with early-stage hepatocellular carcinoma. Clin Chim Acta 2016;462:15-22. [Crossref] [PubMed]
- Chan AW, Kumada T, Toyoda H, et al. Integration of albumin-bilirubin (ALBI) score into Barcelona Clinic Liver Cancer (BCLC) system for hepatocellular carcinoma J Gastroenterol Hepatol 2016;31:1300-6. [Crossref] [PubMed]
- Pinato DJ, Sharma R, Allara E, et al. The ALBI grade provides objective hepatic reserve estimation across each BCLC stage of hepatocellular carcinoma. J Hepatol 2017;66:338-46. [Crossref] [PubMed]


