
A novel insight in the diagnosis of peripheral primitive neuroectodermal tumors: a retrospective analysis of 23 patients
Introduction
Primitive neuroectodermal tumors (PNETs) are rare, small, round cell malignant tumors originating from the neural crest of the neuroectoderm in the central and sympathetic nervous system with multidirectional differentiation potential (1,2). According to its location, PNETs are classified as central PNET (cPNET) or peripheral PNET (pPNET). cPNET refers to a PNET that presents in the cerebral hemisphere, brainstem, cerebellum, or spinal cord. pPNETs usually presents in the torso, limbs, or soft tissue in midline of the body and include Ewing’s sarcoma, which occurs in the bones and soft tissue outside the bones, and Askin tumors, which occur in the chest wall, paraspinal sites, and the retroperitoneal cavity (3-5). However, pPNETs rarely present in other areas (such as the nasal cavity, pancreas, kidney, adrenal gland, intestine, and testis) (6-8). pPNETs are more common in children and adolescents. Although their incidence is very low, including only 4% of all soft tissue tumors (9), pPNETs are associated with a high degree of malignancy, low survival rate, and poor prognosis (3,5). The average median survival is 3.4 years (10). Rapid and accurate diagnosis and assessment of systemic metastases are critical for treatment decision-making and prognosis assessment for pPNET patients.
The clinical manifestations of pPNETs are diverse and nonspecific. Confirmation of the diagnosis depends on histopathology and immunohistochemical results. Noninvasive imaging studies are of great value in the diagnosis and differential diagnosis of pPNETs. However, imaging studies of pPNETs are rare and mainly performed using computed tomography (CT) and magnetic resonance imaging (MRI) (4,6,10-15). Although CT and MRI have obvious advantages in assessing the location, adjacent relationship, and blood supply of the lesion, it is difficult to accurately and rapidly assess the nature of the tumor and to comprehensively evaluate distant metastasis. In recent years, 18F-fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT) has shown unique advantages in the diagnosis, differential diagnosis, staging, and prognosis evaluation of malignant tumors and has been considered an alternative that can compensate for the limitations of CT and MRI (16,17). However, to the best of our knowledge, all 18F-FDG PET/CT imaging studies of pPNET in the current literature are described in case reports (18-25), which represent a lower level of evidence.
This study retrospectively reviewed the clinical data and 18F-FDG PET/CT findings of 23 patients pathologically diagnosed with pPNETs. The results might be valuable for the diagnosis and assessment of pPNET metastasis.
Methods
Patients
The institutional review board of Nanfang Hospital at the Southern Medical University approved the present retrospective study and waived the requirement for written informed consent due to the retrospective nature of the study.
From September 2004 to August 2018, a total of 23 patients with pPNETs who had undergone 18F-FDG PET/CT before the diagnosis was established were enrolled in this study. The diagnosis of pPNET was established by the histopathological and immunohistochemical results. We recorded the age, sex, clinical symptoms, PET/CT findings (the maximum standard uptake value (SUVmax) for lesions, the largest diameter of the tumor, density of lesions, border of tumors, scope of involvement, lymph node metastasis, distant metastasis, etc.) and pathologic results.
18F-FDG PET/CT image examination
Four examinations were performed using a GE Discovery LS PET/CT scanner (GE Healthcare, Waukesha, Wisconsin, USA) and 19 examinations were carried out using a Biograph mCTx scanner (Siemens, Germany). The patients were instructed to fast for at least 6 hours before the PET/CT examination. Approximately 60 minutes after intravenous injection of 5.55 MBq/kg of 18F-FDG, whole-body PET/CT was performed according to the guidelines for tumor imaging with 18F-FDG PET/CT (26) and the established protocols at our center (27).
All PET/CT images were independently read by two nuclear medicine physicians with more than 5 years of experience. The lesions with intense 18F-FDG uptake were considered positive tumors. The regions of interest (ROI) were drawn along the margin of each lesion on the PET images to measure the SUVmax.
Statistical analysis
Statistical analyses were conducted in SPSS Statistics 22. The level of significance was set at α=0.05 (P<0.05), and all tests were 2-tailed. The results for continuous variables were expressed as the mean ± standard deviation, and frequency and ratio were used for ordinal variables. Comparisons between two groups were performed using an independent t-test.
Results
Clinical characteristics of pPNET
Twenty-three patients were included in this study, including 18 males (78.26%). The average age was 26.22±8.50 years (range, 12 to 41 years). Among their symptoms, 16/23 (69.6%) complained of local pain, 4/23 complained of a progressively enlarged mass, 2/23 complained of limb dyskinesia, and 1 patient with a kidney pPNET complained of hematuria.
All patients underwent surgical resection or biopsy. Among them, 9 patients underwent needle biopsy, and 14 underwent surgical resection; 18 patients received chemotherapy, and 9 patients received radiotherapy. As of August 2018, the average follow-up period was 3.5 years (range, 4 weeks to 7 years). The outcomes included death in 11 patients, relapse in 8 patients, and no recurrence in 6 patients.
18F-FDG PET/CT characteristics of pPNET primary lesions
In all 23 pPNET patients, 18F-FDG PET/CT showed a soft tissue mass with hypermetabolism (Table 1), and the detection rate of the tumor was 100%. The maximum diameter of the primary lesion was 7.64±2.39 cm (range, 4.00 to 12.20 cm), and the SUVmax was 11.27±3.80 (range, 5.50 to 21.10). Lesions with invasive growth and ill-defined borders were found in 17/23 (73.91%) patients. Lesions with heterogeneous density and heterogeneous increases in 18F-FDG uptake were noted in 17/23 (73.91%) patients. Necrosis or cystic changes were observed in the lesions of 12/23 (52.17%) patients, and radioactive defects were found at the corresponding sites. No calcification was noted in any of the lesions. The lesions were located in bone [16/23 (69.57%) patients], muscle (2 patients), lung (1 patient), liver (1 patient), kidney (1 patient), testis (1 patient), and mediastinum tissue (1 patient).

Full table
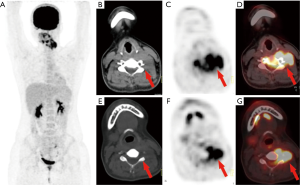
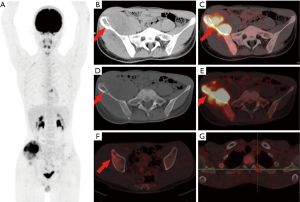
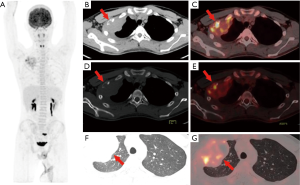
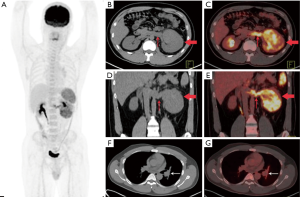
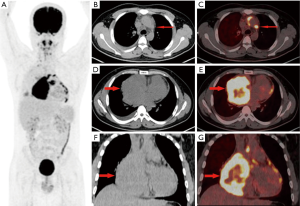
In the 16 patients with pPNETs of bone tissue, the maximum diameter of the lesion was 7.46±2.41 cm (range, 4.30 to 12.20 cm), and the SUVmax was 10.01±3.10 (range, 5.50 to 16.00). Osteolytic bone destruction, osteogenic bone changes, and increased cortical density alone (no obvious bone destruction) were observed in 12/16 (75%), 2/16, and 2/16 patients, respectively. pPNETs of bone tissue were mainly located in the spine and its paravertebral tissue [5/16 patients, the tumor was located in the cervical vertebrae (2 patients), thoracic vertebrae (1 patient), lumbar vertebrae (1 patient), and sacrum (1 patient)], followed by the hip [(4/16 patients, the tumors were located in the ilium (2 patients) and ischium and pubic bone (2 patients)], skull [(3/16 patients, the tumors were located in the mandible (2 patients) and the sphenoid bone (1 patient)], chest wall (i.e., Askin tumor, 3/16 patients), and lower femoral metaphysis (1 patient). In 5 patients with pPNETs in the spine and its paravertebral tissue (Figure 1), the lesions had invaded the spinal canal via the adjacent intervertebral foramen; osteolytic bone destruction was observed in 4 patients, and osteogenic destruction was observed in 1 patient. Osteolytic bone destruction was present in 4 cases of pPNETs of the hip (Figure 2) and 3 cases in the skull. Askin tumors (Figure 3) were present as a single lesion with clear boundaries and compression of adjacent lung tissue in 3 patients, among these patients, osteolytic bone destruction and increased bone cortical density were observed in 2 patients and 1 patient, respectively. In 1 patient with a pPNET in the lower femoral metaphysis, only increased density of cortical bone was noted; no obvious bone destruction was observed.
In the 7 patients with pPNETs arising from non-skeletal organs, the maximum diameter for the primary lesion was 8.07±2.48 cm (range, 5.80 to 11.00 cm) and SUVmax was 14.17±3.87 (range, 10.6 to 21.1). The pPNETs arising from non-skeletal organs had significantly higher FDG uptake than those arising from bones (P=0.012). Among the pPNETs arising from non-skeletal organs, 5/7 showed infiltrated lesions with ill-defined borders, 4/7 showed homogeneous density and increased homogeneous metabolism, and only 2 showed necrosis.
18F-FDG PET/CT characteristics of metastasis in Ppnet
Of the 23 patients, 10 (43.48%) showed metastasis at the time of diagnosis. The detection rate of metastatic lesions by 18F-FDG PET/CT was 100%. Cases with metastasis showed higher FDG uptake (12.53±3.06 vs. 10.31±4.15, P=0.170) than those without. However, they were similar in terms of the maximum diameter of the tumor lesion (8.25±2.72 vs. 7.18±2.11 cm, P=0.297). All cases with metastasis showed hematogenous metastases, including bones (7 cases), bilateral lungs (4 cases), liver (3 cases), vascular tumor thrombus (2 cases), and pericardium (1 case). Lymph node metastasis was observed in 13.0% (3/23) of patients, primarily arising from organs (the primary lesions were in the lung, mediastinum and testis respectively).
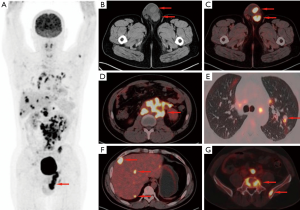
Patients with pPNETs arising from non-skeletal organs had much more metastasis than those with pPNETs arising from bones [6/7 (85.7%) vs. 4/16 (25%)] (Figure 4), and the metastasis mainly involved the lymph nodes (3 cases), bilateral lungs (3 cases), bones (3 cases) and liver (2 cases). One kidney pPNET case showed tumor thrombus in the left renal vein and left pulmonary artery (Figure 5). One liver pPNET case showed multiple intrahepatic metastases and inferior vena cava tumor thrombus. One pPNET case arising from the mediastina showed lymph node metastasis and multiple pericardial metastases (Figure 6).
Pathological characteristics of pPNET
The pPNET lesions were composed of uniform small round cells with deep nuclear staining and obvious mitosis. The tumor cells were diffuse or scattered, and some were arranged into Homer-Wright rosettes. Immunohistochemical analysis of the 23 pPNET lesions revealed that that 22 were positive for CD99, and the positive rates of Fli, Vim (vimentine), Syn (synaptophysin), NSE (neurospecific enolase) and CgA (chromogranin A) were 65.22% (15/23), 56.52% (13/23), 56.52% (13/23), 47.83% (11/23) and 21.74% (5/23), respectively. Two groups were defined based on a Ki67 proliferation index cutoff of 50%. A high Ki67 index (Ki67 ≥50%) accounted for 56.52% (13/23), and a low Ki67 index (Ki67 <50%) accounted for 43.48% (10/23). The pPNET primary lesions with a high Ki67 index showed higher FDG uptake (12.74±4.01 vs. 9.37±2.61, P<0.05).
Discussion
Clinical and pathological characteristics of pPNETs
pPNETs are more common in children and adolescents (5,6,10,11). In this study, most of the patients were young men (the mean age was 26.22 years, and males accounted for 78.26% of all patients). Age differences among pPNET patients may be associated with differences in the origin of the tumor (12). The most common primary site of pPNETs was bone tissue (16/23, 69.57%), followed by the muscles (2 patients), lungs (1 patient), liver (1 patient), kidneys (1 patient), testis (1 patient), and mediastinum (1 patient). pPNETs of bone tissue were more common in the spine and paravertebral tissue, followed by the hip, chest wall (Askin tumor), and skull. The primary site of pPNETs in this study is consistent with previous studies (10,11,14,15).
pPNETs belong to the family of malignant small round cell tumors, and their diagnosis depends on pathological and immunohistochemical results. The characteristic structure of a pPNET is Homer-Wright rosettes under the microscope (28). CD99, the product of MIC gene, is a relatively specific antibody for pPNET family tumors. In this study, the positive rate of CD99 was as high as 95%, whereas other markers of neural differentiation characteristics (such as Vim, Syn, NSE, CgA) showed various expression levels, consistent with previous studies (13,29).
18F-FDG PET/CT characteristics of pPNET primary and metastatic lesions
18F-FDG PET/CT is a new type of noninvasive imaging technique that has been widely used in the diagnosis of malignant tumors (30-32). 18F-FDG is a glucose analog. The uptake of 18F-FDG by tumors can reflect the glucose metabolism, levels of glucose transporters (especially GLU-1 and GLU-3), and the expression level of hexokinase in the tumor (33,34). To date, the 18F-FDG PET/CT imaging features of pPNETs have only been published in case reports (18-25) (see Table 2 for a summary). A soft tissue mass with high uptake of 18F-FDG and the SUVmax ranging from 4.36 to 17.2 is observed in most pPNET lesions (18-24). Significantly high 18F-FDG uptake by pPNET lesions was observed in all 23 patients reported in this study. The SUVmax was 11.27±3.80 (range, 5.50 to 21.10), suggesting abnormally increased glycolysis in pPNET lesions. This study analyzed the correlation between the SUVmax and the Ki67 index (i.e., the tumor cell proliferation index) of pPNETs and found that a high Ki-67 index of pPNETs was associated with increased 18F-FDG uptake. This result further suggests that the increased metabolism of pPNETs reflect their extremely active proliferation and high degree of malignancy. However, high uptake of 18F-FDG may be not observed in all pPNET lesions. Musana et al. reported the absence of 18F-FDG uptake in a patient with a pPNET in the right arm and lung metastasis (25). This finding may be due to differences in the biological behavior and metabolism of pPNETs in different organs. In this particular case, the pPNET may exhibit low expression levels of GLU-1 and/or hexokinase II.

Full table
pPNETs are prone to early metastasis, which was observed in 43.48% (10/23) of patients in this study at the time of diagnosis. Lymph node metastasis of pPNETs is not common (15,18,24), and hematogenous metastasis to bone, lung, and liver tissue (4,11,25) is more prevalent and may cause vascular tumor thrombus (8,20) in some cases. Lymph node metastasis was reported in only 3 patients in this study. In contrast, hematogenous metastasis was observed in 10 patients, and bone tissue (7/23) was mainly involved, followed by the lungs (4/23), liver (3/23), vascular tumor thrombus (2/23) and the pericardium (1/23). Lymph node metastasis was observed in only 13.0% of patients. This study revealed that the metabolic rate of non-skeletal primary pPNETs was significantly higher than that of pPNETs of bone tissue, and the incidence of metastasis of the former was significantly higher than that of the latter (85.7% vs. 25%). This finding strongly suggests that non-skeletal pPNETs are more malignant, proliferative, and prone to distant metastasis.
Advantages of 18F-FDG PET/CT vs. CT or MRI
Previously, CT and MRI were the major methodologies for imaging studies of pPNETs (4,6,10-15). Both CT and MRI have shown that pPNETs are manifested as soft tissue masses with heterogeneous density, ill-defined borders, and cystic or necrotic areas and invasion into adjacent normal tissues (4,6,10-15). Calcification is very rare (11,13). MRI shows an isointense or hypointense mass on T1 weighted imaging (WI) and a hyperintense mass on T2WI (4,6,10,13). Enhanced CT and T1WI display heterogeneous enhancement of the mass (4,6,10,12-14). In this study, 18F-FDG PET/CT showed that the primary pPNET lesion was a large soft tissue mass (average diameter, 7.64 cm); 73.9% of lesions showed ill-defined borders, heterogeneous density, and invasion into adjacent tissues, and 52.17% of lesions showed cystic changes or necrosis. No lesions exhibited calcification. Therefore, PET/CT is not inferior to CT and MRI in terms of identifying the location, extent, boundary, density, presence or absence of cystic/necrosis, and relationship with adjacent tissues in the primary lesions. However, PET/CT can more intuitively reflect the biological behavior of the tumor due to varying degrees of 18F-FDG uptake in pPNET primary lesions and has advantages for the detection rate of the lesions and the determination of benign and malignant tumors.
In addition, 18F-FDG PET/CT allows whole-body imaging and is significantly better than CT and MRI for the detection of distant metastases. In particular, rapid and accurate detection of metastatic lesions has important clinical value in the treatment of pPNETs because they exhibit a high degree of malignancy and early metastasis. In this study, at the time of diagnosis, metastatic pPNETs were identified in 10 patients by 18F-FDG PET/CT, which had an important impact on treatment decisions.
Additionally, 18F-FDG PET/CT can simultaneously display the metabolic rates of primary and metastatic pPNET lesions and play an important role in the efficacy evaluation and follow-up (20,21). In this study, 5 patients with pPNETs underwent 18F-FDG PET/CT for systemic examination after surgery and/or chemoradiotherapy (between 3 months and 2 years). No obvious signs of recurrence were noted in 2 patients. The volume and FDG uptake of primary or metastatic lesions was significantly reduced in 2 patients, suggesting that the tumors were significantly suppressed or inactivated. One patient had obvious signs of recurrence and was treated with re-operation and repeat chemotherapy.
Conclusions
The significant characteristics of 18F-FDG PET/CT imaging of pPNETs include primary lesions are soft tissue masses with a significantly high FDG uptake and ill-defined borders, heterogeneous density, and invasion into adjacent tissues. Necrosis or cystic changes are observed in some cases, and lesions are free of calcification. pPNETs are prone to early metastasis. The 18F-FDG uptake of metastatic lesions is significantly increased. Hematogenous metastasis to bone, lung, and liver tissue is common, but lymph node metastasis is rare. 18F-FDG PET/CT can easily detect pPNETs, but the final diagnosis relies on pathological and immunohistochemical examinations. However, 18F-FDG PET/CT can clearly show the location, extent, boundary, density, presence or absence of cystic changes/necrosis, relationship with adjacent tissues and organs, and metabolic status of the primary lesions; thus, it can be used to comprehensively evaluate lymph node and distant metastasis. Compared to traditional CT imaging and MRI, 18F-FDG PET/CT has significant advantages for assessing the possibility of tumor resection, degree of systemic metastasis, therapeutic efficacy, postoperative residual tumor, and recurrence.
Acknowledgments
Funding: This work was supported by the a President Foundation Project of Nanfang Hospital, Southern Medical University [grant number 2015C027], a Natural Science Foundation of Guangdong Province [grant number 2016A030310398] and a National Natural Science Foundation [grant number 81501511].
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.12.24). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The institutional review board of Nanfang Hospital at the Southern Medical University approved the present retrospective study and waived the requirement for written informed consent due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dehner LP. Peripheral and central primitive neuroectodermal tumors. A nosologic concept seeking a consensus. Arch Pathol Lab Med 1986;110:997-1005. [PubMed]
- Hart MN, Earle KM. Primitive neuroectodermal tumors of the brain in children. Cancer 1973;32:890-7. [Crossref] [PubMed]
- Schmidt D, Herrmann C, Jurgens H, et al. Malignant peripheral neuroectodermal tumor and its necessary distinction from Ewing's sarcoma. A report from the Kiel Pediatric Tumor Registry. Cancer 1991;68:2251-9. [Crossref] [PubMed]
- Winer-Muram HT, Kauffman WM, Gronemeyer SA, et al. Primitive neuroectodermal tumors of the chest wall (Askin tumors): CT and MR findings. AJR Am J Roentgenol 1993;161:265-8. [Crossref] [PubMed]
- Jürgens H, Bier V, Harms D, et al. Malignant peripheral neuroectodermal tumors. A retrospective analysis of 42 patients. Cancer 1988;61:349-57. [Crossref] [PubMed]
- Khong PL, Chan GC, Shek TW, et al. Imaging of peripheral PNET: common and uncommon locations. Clin Radiol 2002;57:272-7. [Crossref] [PubMed]
- Czekalla R, Fuchs M, Stolzle A, et al. Peripheral primitive neuroectodermal tumor of the stomach in a 14-year-old boy: a case report. Eur J Gastroenterol Hepatol 2004;16:1391-400. [Crossref] [PubMed]
- Dong J, Xing J, Limbu HH, et al. CT Features and Pathological Correlation of Primitive Neuroectodermal Tumor of the Kidney. Cell Biochem Biophys 2015;73:59-64. [Crossref] [PubMed]
- Coffin CM, Dehner LP. Peripheral neurogenic tumors of the soft tissues in children and adolescents: a clinicopathologic study of 139 cases. Pediatr Pathol 1989;9:387-407. [Crossref] [PubMed]
- Ibarburen C, Haberman JJ, Zerhouni EA. Peripheral primitive neuroectodermal tumors. CT and MRI evaluation. Eur J Radiol 1996;21:225-32. [Crossref] [PubMed]
- Dick EA, McHugh K, Kimber C, et al. Imaging of non-central nervous system primitive neuroectodermal tumours: diagnostic features and correlation with outcome. Clin Radiol 2001;56:206-15. [Crossref] [PubMed]
- Li X, Zhang W, Song T, et al. Primitive neuroectodermal tumor arising in the abdominopelvic region: CT features and pathology characteristics. Abdom Imaging 2011;36:590-5. [Crossref] [PubMed]
- Tan Y, Zhang H, Ma GL, et al. Peripheral primitive neuroectodermal tumor: dynamic CT, MRI and clinicopathological characteristics--analysis of 36 cases and review of the literature. Oncotarget 2014;5:12968-77. [Crossref] [PubMed]
- Qian X, Kai X, Shaodong L, et al. Radiological and clinicopathological features of pPNET. Eur J Radiol 2013;82:e888-93. [Crossref] [PubMed]
- Gong J, Zhang Y, Zuo M, et al. Imaging findings of abdominal peripheral primitive neuroectodermal tumor: report of four cases with pathological correlation. Clin Imaging 2009;33:196-9. [Crossref] [PubMed]
- Jadvar H, Colletti PM, Delgado-Bolton R, et al. Appropriate Use Criteria for (18)F-FDG PET/CT in Restaging and Treatment Response Assessment of Malignant Disease. J Nucl Med 2017;58:2026-37. [Crossref] [PubMed]
- Meignan M, Itti E, Gallamini A, et al. FDG PET/CT imaging as a biomarker in lymphoma. European journal of nuclear medicine and molecular imaging. 2015;42:623-33. [Crossref] [PubMed]
- Yu P, Xiaochun F, Jing L, et al. Rare Ileal Ewing Sarcoma/Primitive Neuroectodermal Tumor on 18F-FDG PET/CT. Clin Nucl Med 2017;42:809-11. [Crossref] [PubMed]
- Dong A, Wang Y, Lu J, et al. FDG PET/CT in peripheral primitive neuroectodermal tumor of the retroperitoneum. Clin Nucl Med 2014;39:707-10. [Crossref] [PubMed]
- Aras M, Dede F, Dane F, et al. FDG PET/CT appearance of portal vein tumor thrombus in the gastric primitive neuroectodermal tumor: uncommon primary tumor site with rare finding. Clin Nucl Med 2013;38:47-9. [Crossref] [PubMed]
- Mao L, Wang H, Xie G, et al. Rare pulmonary primitive neuroectodermal tumor metastasizing to the right atrium: a case report. Med Oncol 2012;29:2649-53. [Crossref] [PubMed]
- Kara Gedik G, Sari O, Altinok T, et al. Askin's Tumor in an Adult: Case Report and Findings on 18F-FDG PET/CT. Case Rep Med 2009;2009:517329 [Crossref] [PubMed]
- Demir MK, Kosar F, Sanli Y, et al. 18F-FDG PET-CT features of primary primitive neuroectodermal tumor of the chest wall. Diagn Interv Radiol 2009;15:172-5. [PubMed]
- Kamaleshwaran KK, Mittal BR, Chakraborty D, et al. Imaging with 18F-FDG PET/CT of a primitive primary neuroectodermal tumor of the chest wall, in an adult. Hell J Nucl Med 2010;13:287-8. [PubMed]
- Musana KA, Raja S, Cangelosi CJ, et al. FDG PET scan in a primitive neuroectodermal tumor. Ann Nucl Med 2006;20:221-5. [Crossref] [PubMed]
- Delbeke D, Coleman RE, Guiberteau MJ, et al. Procedure guideline for tumor imaging with 18F-FDG PET/CT 1.0. J Nucl Med 2006;47:885-95. [PubMed]
- Zhou WL, Wu HB, Wang LJ, et al. Usefulness and pitfalls of F-18-FDG PET/CT for diagnosing extramedullary acute leukemia. Eur J Radiol 2016;85:205-10. [Crossref] [PubMed]
- Brinkhuis M, Wijnaendts LC, van der Linden JC, et al. Peripheral primitive neuroectodermal tumour and extra-osseous Ewing's sarcoma; a histological, immunohistochemical and DNA flow cytometric study. Virchows Arch 1995;425:611-6. [Crossref] [PubMed]
- de Alava E, Gerald WL. Molecular biology of the Ewing's sarcoma/primitive neuroectodermal tumor family. J Clin Oncol 2000;18:204-13. [Crossref] [PubMed]
- Pawlik TM. Intrahepatic cholangiocarcinoma: from diagnosis to treatment. Hepatobiliary Surg Nutr 2017;6:1. [Crossref] [PubMed]
- Fábrega-Foster K, Ghasabeh MA, Pawlik TM, et al. Multimodality imaging of intrahepatic cholangiocarcinoma. Hepatobiliary Surg Nutr 2017;6:67-78. [Crossref] [PubMed]
- Li C, Li R, Zhang W. Progress in non-invasive detection of liver fibrosis. Cancer Biol Med 2018;15:124-36. [Crossref] [PubMed]
- Yoon SO, Jeon TJ, Park JS, et al. Analysis of the roles of glucose transporter 1 and hexokinase 2 in the metabolism of glucose by extrahepatic bile duct cancer cells. Clin Nucl Med 2015;40:e178-82. [Crossref] [PubMed]
- Kang F, Ma W, Ma X, et al. Propranolol inhibits glucose metabolism and 18F-FDG uptake of breast cancer through posttranscriptional downregulation of hexokinase-2. J Nucl Med 2014;55:439-45. [Crossref] [PubMed]


