
Therapeutic effects of different regimens for induction therapy in elderly patients more than 50 years old with newly diagnosed acute myeloid leukaemia
Introduction
Acute myeloid leukaemia (AML) is the most common malignancy of the blood and is highly heterogeneous. The incidence increases with age (1-3), and age is an independent prognostic factor for AML (1,4-6). Despite the advances in the treatment of AML (7), elderly patients still experience poor prognosis due to poor performance status and comorbidities and are more likely to carry a naturally drug-resistant gene. Elderly patients who achieve complete remission are faced with short survival time and high relapse rates. At present, there remains no standard induction therapy for elderly patients with AML, and they are suggested to participate in clinical trials. Many studies have shown that hypomethylating agents, such as decitabine, are an effective treatment option for elderly AML patients, especially in poor-risk cohorts (5,8). Several low-intensity decitabine-based chemotherapy regimens have emerged gradually. This study retrospectively analysed three commonly used regimens to provide evidence for clinical treatment.
Methods
Patients and inclusion criteria
A total of 86 newly diagnosed AML (non-M3) patients who were admitted between September 01, 2012 and June 30, 2017 in the First Affiliated Hospital of Chongqing Medical University and the Second Affiliated Hospital of Chongqing Medical University were included in this study. The inclusion criteria were follows: (I) patients aged ≥50 years; (II) patients who met the diagnostic criteria for AML, that is to say, those who had a bone marrow or peripheral blood blast cell count of ≥20% (9); (III) patients who had an Eastern Cooperative Group performance status (ECOG PS) score of <3; (IV) patients who did not develop severe complications in other organs.
Treatment
The patients were divided into three groups based on the treatment received. Among them, 19 were included in the IA group, 36 in the D-CAG group, and 31 in the DA group. The IA regimen was consists of a 3+7 course of idarubicin 10–20 mg/d given for 3 days (d1–d3) and cytarabine 100–150 mg/d given for 7 days (d1–d7). DA regimen was conducted by administering 35–60 mg/m2 of daunorubicin, as a substitute for idarubicin on the basis of the IA regimen, for 3 days (d1–d3). D-CAG regimen was conducted by giving 25 mg/d of decitabine for 5 days, 30-40 mg/d of cytarabine for 4 days, 10 mg/d of aclarubicin for 7 days, and 300 μg/d of G-CSF from day 1 until the WBC count increased to more than 20×109/L. After remission, most patients in D-CAG group received one to three courses of D-CAG regimen for consolidation therapy, while patients in the IA and DA group receive one or two courses of the same regimen followed by high dose cytarabine. Patients with residual disease after the first cycle of induction were allowed to receive a second induction cycle.5 patients in IA group, 3 in D-CAG group, and 7 in DA group received hematopoietic stem cell transplantation (HSCT), of whom all but five were younger than 60 years old.
Supportive treatment
Patients with a haemoglobin level of ≤70 g/L were infused with red blood cells. By contrast, patients with a platelet count of ≤20×109/L received platelet transfusion. In addition, antibiotics were used to prevent and treat infections.
Response criteria and adverse reactions criteria
The therapeutic efficacy was evaluated after one course of induction treatment. The treatment outcomes were divided into early death, complete remission (CR), partial remission (PR), and no remission (NR). The overall response (OR) rate was computed by adding the CR rate and PR rate. Early death was defined as death within 30 days from the first day of induction chemotherapy. Patients who achieved CR had a bone marrow blast of less than 5% and haemoglobin count of >100 g/L (male) or 90 g/L (female), neutrophil count of >1.5×109/L, and platelet count of >100×109/L in the peripheral blood. Patients who achieved PR had a bone marrow blast of 5–20% or their clinical manifestation and peripheral blood count did not fully meet the criteria for CR. Patients were classified as NR when their bone marrow blasts, peripheral blood blasts, and clinical manifestations did not meet the criteria for CR. Adverse reactions were defined based on the World Health Organization’s (WHO) chemotherapy side effects criteria.
Statistical analysis
All data were analysed using the Statistical Program for Social Sciences version 22.0. The measurement data were analysed using t-test and rank sum test, while enumeration data were analysed using chi-square test. The Kaplan-Meier method was used to perform survival analysis, log-rank test to compare the survival rates, and Cox regression model to identify the factors influencing survival. A P value of <0.05 implied a significant difference.
Results
Therapeutic evaluation
The baseline characteristics of the three groups were compared (Table 1). There was no significant difference between the IA group and the DA group. Moreover, no significant difference was observed in the sex ratio, ECOG score, infection rates, and haemorrhage rates before chemotherapy of the three groups. However, the median age of the D-CAG group were higher than that of the IA group and DA group while the median bone marrow blasts and WBC count were lower.
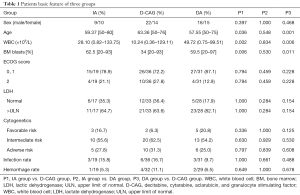
Full table
All 86 patients completed one course of induction chemotherapy. However, the efficacy of this therapy was not evaluated in one patient from the D-CAG group and three from the DA group as the results of their bone marrow exam were not reviewed. There were 7 patients in IA group, 22 in D-CAG group, 6 in DA group received two courses of induction, the rest received just one course. The early death rates, CR rates, and OR rates of the three groups showed no significant difference (Table 2).

Full table
Adverse reactions
All patients developed grade IV haematological toxicities. The grade IV adverse reactions experienced by the patients and the appearing time of the lowest value of patients’ WBC, platelet, and haemoglobin were compared among the three groups.
Furthermore, we compared the time required for the WBC values to recover to ≥1×109/L, for the platelet values to recover to ≥20×109/L, and for the haemoglobin values to recover to ≥60 g/L. Among them, the incidence of grade IV WBC toxicity in the D-CAG group was lower than that in the IA and DA group (Table 3).
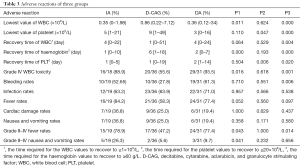
Full table
Compared with the DA group, the lowest values of WBC and platelet were higher in D-CAG group and the recovery time of WBC and platelet was shorter. However, the time required for haemoglobin to recover to ≥60 g/L in the D-CAG group was longer than that in the DA group. Compared with the IA group, the lowest WBC count after chemotherapy was higher in the D-CAG group but the time required for haemoglobin values to recover was longer. The IA group had similar hematologic adverse reactions with the DA group, except that the IA group had the lowest platelet value after chemotherapy and the time required for platelet values to recover was shorter in the IA group than that in the DA group (Table 3). No statistical difference was observed in other blood system adverse reactions of all three groups.
The primary non-haematological adverse reactions experienced by the patients were infection, fever, gastrointestinal symptoms, bleeding, and cardiac damage. Cardiac damage was primarily manifested by arrhythmia and acute cardiac insufficiency. The incidence of grade II–IV fever was lower in the D-CAG group than in the DA group and IA group. The D-CAG group had lower incidence of bleeding than the DA group and had lower incidence of grade II–IV nausea and vomiting than the IA group (Table 3). There was no statistical difference in non-hematologic adverse reactions between the DA group and the IA group.
Survival follow-up
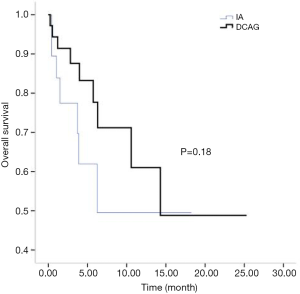
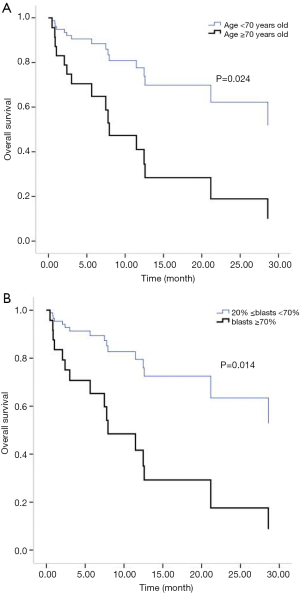
All patients were followed up until March 31, 2018. Two cases of early death occurred in both the IA group and the D-CAG group. Among them, one died of suicide and the other died of cerebral haemorrhage both in the two groups. There was no case of early death in the DA group. For the survival follow-up of the IA group and the D-CAG group, the median survival times were 12.47 months and 28.6 months, respectively (P=0.18) (Figure 1). Cox regression analysis showed that the age of ≥70 years and a bone marrow blasts of ≥70% at initial diagnosis had a negative effect on long-term survival (Figure 2). However, the study showed that WBC count, LDH level, and ECOG score had no influence on the long-term survival.
Discussion
In the clinical treatment course of AML, most patients age 50–60 have bad situation and they can not tolerate intensive chemotherapy. And a few studies also choose ≥50 years old as the age limits (10,11).
In this study, the basic characteristics of patients in the IA and DA group were similar and comparable. Except that the IA group had a lower incidence of platelet toxicity while the time required for the haemoglobin values to recover to ≥60 g/L was longer than the DA group; there was no statistical difference between the two groups in terms of other haematological or non-haematological adverse reactions. In addition, there were no statistical difference in early death rates, CR rates, or OR rates between the two groups. It seems that the IA and DA regimens have similar therapeutic efficacy and adverse reactions to elderly AML patients. Hence, further studies should be conducted to explore the long-term effects of these regimens. A few studies pointed that the dose of daunorubicin remains controversial. However, for elderly fit patients, giving a 60 or 90 mg/m2 dose may result in higher CR rates and longer survival time than giving a 45 mg/m2 dose. However, compared with 60 mg/m2 dose, administering a 90 mg/m2 dose may not provide further benefits to the patients. On the contrary, it may cause more adverse reactions (12,13). The Chinese guidelines suggests that the standard dose is 40–60 mg/m2 for elderly fit patients (14).
The semi-annual relapse rates of IA, D-CAG and DA group were 37.5% (3/8), 13.3% (2/15) and 40% (4/10), respectively. Although the relapse rate was lower of D-CAG group, there was no statistical difference in three groups (P=0.247). The median OS time of the IA and D-CAG group were 12.47 months and 28.6 months, respectively, and no statistical difference was observed. However, as was shown in Figure 1, it seemed like there was a trend that patients of D-CAG group had a better long-term survival. Even so, the survival time was still too short for these patients. It was clear that age was an independent risk factor for AML patients. Our study also showed the same result. Moreover, age was included and listed separately by many prognostic models (6,15,16). Same as some other studies, we found high bone marrow blasts at initial diagnosis was a poor prognostic factor (17).
When we compared the baseline characteristics, we found that the median age of the D-CAG group was higher than that of the IA group and DA group while the median bone marrow blasts and WBC count were lower. Increasing the sample volume may eliminate these differences, and this is our future direction. As age was a generally accepted independent risk factor, it seemed that the D-CAG group had a worse physical condition than the IA and DA groups. Due to the limitations related to the fact that this is a retrospective study, it is possible that the physicians chose more sick patients for D-CAG regimen. In this condition, three group had similar CR rates. Moreover, the D-CAG group had lesser haematological and non-haematological adverse reactions than the IA and DA groups, especially in the haematological system. This finding indicated that the D-CAG regimen maybe a more effective treatment that can help achieve a complete remission with less adverse reaction. However, large sample studies are still needed. Our study showed that D-CAG group had less myelosuppression, decreasing the incidence of grade II–IV fever, which may be due to the use of G-CSF in the D-CAG regimen. Bob pointed that G-CSF can enhance the efficacy of a chemotherapy regimen by deriving leukemia cells into the cell cycle to increase the susceptibility of the cells to be killed by cytarabine (18). In fact, during our data collection, we found that the D-CAG regimen was used more frequently than the other two regimens gradually.
The CR rate was calculated after one course of induction treatment, which resulted in it being lower than usual. At present, increasing the CR rate of elderly AML patients remains a concern that needs to be addressed. Although the current treatment outcome is considered less desirable, receiving chemotherapy is a better option (3,19,20). As AML is a heterogeneous disease and there is no standard criteria in choosing the best treatment, a comprehensive and systematic assessment of elderly patients before chemotherapy will have a significant effect on the patients’ treatment outcome, and many prognostic models can be applied (6,15,16). Allogeneic hematopoietic stem cell transplantation remains the primary treatment for patients to achieve long-term remission; finding new target drugs may be beneficial to these patients. We do expect that the incorporation of newer therapies might change or impact these outcomes, such as the addition of FLT3 inhibitors, the use of Vyxeos (liposomal daunorubicin/cytarabine) for secondary AML in older patients, and venetoclax which has been studied in older AML patients with quite promising data.
Conclusions
Newly diagnosed AML patients aged ≥50 years are still faced with low complete remission rate and poor long-term survival conditions. Age and bone marrow blasts at initial diagnosis are risk factors for prognostic. The IA, D-CAG, and DA regimens as induction therapies can be well tolerated. D-CAG regimen can be a better treatment option as it only causes less adverse reactions and patients may have better long-term survival. Studies with larger sample size should be conducted. Hence, substantial efforts are needed to explore new drugs and treatments to benefit these patients.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (no. 81772280).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.12.21). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The written informed consent was waived due to the retrospective nature of the study. This study was approved by the ethics committee of Yongchuan Hospital of ChongQing Medical University.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Koller E. Treatment of elderly and very old patients with AML. Memo 2016;9:4-7. [Crossref]
- Forman D, Stockton D, Moller H, et al. Cancer prevalence in the UK: results from the EUROPREVAL study. Ann Oncol 2003;14:648-54. [Crossref] [PubMed]
- Juliusson G, Antunovic P, Derolf A, et al. Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood 2009;113:4179-87. [Crossref] [PubMed]
- Mrozek K, Marcucci G, Nicolet D, et al. Prognostic significance of the European LeukemiaNet standardized system for reporting cytogenetic and molecular alterations in adults with acute myeloid leukemia. J Clin Oncol 2012;30:4515-23. [Crossref] [PubMed]
- Quintas-Cardama A, Ravandi F, Liu-Dumlao T, et al. Epigenetic therapy is associated with similar survival compared with intensive chemotherapy in older patients with newly diagnosed acute myeloid leukemia. Blood 2012;120:4840-5. [Crossref] [PubMed]
- Wheatley K, Brookes CL, Howman AJ, et al. Prognostic factor analysis of the survival of elderly patients with AML in the MRC AML11 and LRF AML14 trials. Br J Haematol 2009;145:598-605. [Crossref] [PubMed]
- Burnett AK. Treatment of acute myeloid leukemia: are we making progress? Hematology 2012;2012:1-6. [PubMed]
- Blum W, Garzon R, Klisovic RB, et al. Clinical response and miR-29b predictive significance in older AML patients treated with a 10-day schedule of decitabine. Proc Natl Acad Sci U S A 2010;107:7473-8. [Crossref] [PubMed]
- Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia. Blood 2016;127:2391. [Crossref] [PubMed]
- Pautas C, Thomas X, Merabet F, et al. Randomized Comparison of Standard Induction with Daunorubicin (DNR) for 3 Days vs Idarubicin (IDA) for 3 or 4 Days in AML pts Aged 50 to 70 and of Maintenance with Interleukin 2. Final Analysis of the ALFA 9801 Study. Blood 2018;110:162.
- Stefan F, Srdan V, Jorge C, et al. Clofarabine and cytarabine combination as induction therapy for acute myeloid leukemia (AML) in patients 50 years of age or older. Blood 2006;108:45. [Crossref] [PubMed]
- Saultz JN, Garzon R. Acute Myeloid Leukemia: A Concise Review. J Clin Med 2016;5:33. [Crossref] [PubMed]
- Burnett AK, Russell NH, Hills RK, et al. A randomized comparison of daunorubicin 90 mg/m2 vs 60 mg/m2 in AML induction: results from the UK NCRI AML17 trial in 1206 patients. Blood 2015;125:3878-85. [Crossref] [PubMed]
- Leukemia & Lymphoma Group, Chinese Society of Hematology, Chinese Medical Association. Chinese guidelines for diagnosis and treatment of adult acute myeloid leukemia (not APL) (2017). Zhonghua Xue Ye Xue Za Zhi 2017;38:177-82. [PubMed]
- Koca E, Halacoglu A, Malkan UY, et al. Prognostic Factors for Survival of Elderly Patients with Acute Myeloid Leukemia after Intensive Chemotherapy: Validation of 3 Popular Prognostic Models. Blood 2014;124:5332.
- Christoph RL, Christian T, Martin G, et al. A novel prognostic model in elderly patients with acute myeloid leukemia: results of 909 patients entered into the prospective AML96 trial. Blood 2010;116:971. [Crossref] [PubMed]
- Chen Y, Yang T, Zheng X, et al. The outcome and prognostic factors of 248 elderly patients with acute myeloid leukemia treated with standard-dose or low-intensity induction therapy. Medicine 2016;95:e4182 [Crossref] [PubMed]
- Bob LW, Wim VP, Matthias T, et al. Effect of priming with granulocyte colony-stimulating factor on the outcome of chemotherapy for acute myeloid leukemia. N Engl J Med 2003;349:743. [Crossref] [PubMed]
- Löwenberg B, Zittoun R, Kerkhofs H, et al. On the value of intensive remission-induction chemotherapy in elderly patients of 65+ years with acute myeloid leukemia: a randomized phase III study of the European Organization for Research and Treatment of Cancer Leukemia Group. J Clin Oncol 1989;7:1268-74. [Crossref] [PubMed]
- Oran B, Weisdorf DJ. Survival for older patients with acute myeloid leukemia: a population-based study. Haematologica 2012;97:1916-24. [Crossref]


