Axitinib as first-line therapy in metastatic renal cell carcinoma
Axitinib is a potent and selective second-generation VEGFR inhibitor (1). A pivotal phase III trial, named AXIS, revealed the superiority of axitinib over sorafenib in terms of progression-free survival (PFS) in advanced renal cell carcinoma (RCC) after failure of one previous systemic therapy (2). In the AXIX trial, median PFS in cytokine-refractory patients was 12.1 months with axitinib versus 6.5 months with sorafenib.
However, Hutson et al. recently reported a negative result from a relatively small-numbered phase III trial in which 288 treatment-naïve patients with metastatic RCC were randomly assigned to axitinib and sorafenib (3). The trial was designed with the prediction of 4.3 months improvement in median PFS in axitinib over sorafenib. The design looked reasonable because previous trials showed median PFS of 12.1 to 13.7 months with axitinib in cytokine-refractory disease, on the other hand, median PFS of 5.5 to 6.5 months with sorafenib in cytokine-refractory or treatment-naïve patients (2,4-6). As a result, median PFS with sorafenib was not surprising, whereas median PFS with axitinib was disappointing. There was no significant difference in median PFS between patients treated with axitinib and sorafenib in this trial [10.1 months (95% CI, 7.2-12.1) vs. 6.5 months (95% CI, 4.7-8.3)], respectively; stratified hazard ratio 0.77 (95% CI, 0.56-1.05) (3). One of the reasons for this result may be that more patients without previous nephrectomy were included in the axitinib group (15%) than in the sorafenib group (10%). Indeed, a subgroup analysis of patients with previous nephrectomy showed that median PFS was significantly improved with axitinib versus sorafenib. However, it was noteworthy that median PFS in patients with ECOG performance status 1 was quite similar between the axitinib group and the sorafenib [6.5 months (95% CI, 3.7-8.4) vs. 6.4 months (95% CI, 4.4-11.1)], respectively; hazard ratio 0.93 (95% CI, 0.59-1.48), although median PFS was significantly longer with axitinib versus sorafenib in patients with ECOG performance status 0 [13.7 months (95% CI, 10.1-19.4) vs. 6.6 months (95% CI, 4.7-9.9), respectively]; hazard ratio 0.64 (95% CI, 0.42-0.99).
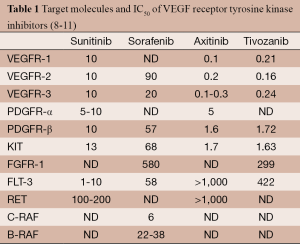
A same trend was also observed in the TIVO-1 trial, in which tivozanib and sorafenib were compared as initial targeted therapy for metastatic RCC (7). Patients with ECOG performance status 0 treated with tivozanib had significantly longer median PFS than those with sorafenib (14.8 vs. 9.1 months, respectively; P=0.004), however, no difference was observed between the tivozanib group and the sorafenib in patients with ECOG performance status 1 (9.1 vs. 9.0 months, respectively; P=0.59). Median PFS with sorafenib was similar in patients with ECOG performance status 0 and 1 in both TIVO-1 and this axitinib trial. Such steady treatment effect of sorafenib regardless of performance status can be advantage over other targeted agents for advanced RCC. In contrast, the effect of axitinib and tivozanib may be affected by patient’s performance status. Axitinib and tivozanib have a similar target profile that is relatively specific to VEGFR-1, 2, and 3 (Table 1) and this characteristics common between these two agents may have contributed to the trend as to treatment effect and performance status.
In this axitinib study, both agents were given according to their approved labeling, such that dose increases were allowed with axitinib, but not with sorafenib. This difference in dose increase capability may have made a contribution to the difference in objective response rates between axitinib and sorafenib [32% vs. 15%, respectively; risk ratio 2.21 (95% CI, 1.31-3.75)], as well as to the rates of serious adverse events (34% vs. 25%, respectively). All major adverse events in all grades but plantar erythrodysaesthesia and rash were also more common with axitinib versus sorafenib. Disease progression was the most common cause of death in both groups (86% in axitinib and 97% in sorafenib), however 14% of patients in the axitinib group died of other reasons though the detail was not described except one treatment-related cardiac arrest.
Median duration of response was 14.7 months with axitinib and 14.3 months with sorafenib, that means there was no difference in the time to evolution of resistance against each drug.
Overall, this axitinib study was underpowered for its primary endpoint and it is questionable why sorafenib was chosen as the control in first-line setting although the authors explained the reason as sorafenib was available in the regions where the trial was conducted. Sunitinib or pazopanib definitely should be used as a comparator in the treatment-naïve setting. However, what is suggested from this study is that PFS with axitinib as first-line therapy will not demonstrably overwhelm that with sunitinib or pazopanib. The PFS of tivozanib, another highly potent and selective VEGFR inhibitor, was also similar with these three drugs based on TIVO-1 trial. It suggests that median PFS with VEGF signal-targeted drugs is around 10 months in first-line setting. So another trial with a larger sample size of axitinib and sorafenib or with sunitinib or pazopanib as a comparator in first-line setting, if planed, will not be intriguing. In the future, development of absolutely novel targeted therapy for this disease is highly desired. Combination of a new agent and an existing drug can be also interesting.
Although PFS is the most commonly used surrogate endpoint for clinical trials in metastatic RCC, the correlation of PFS and overall survival (OS) still remains unclear. Recent phase III trials, including INTORSECT and TIVO-1, also showed unique PFS and OS data. The INTORSECT trial, a head-to-head phase III trial comparing temsirolimus and sorafenib in second-line therapy, showed that PFS was slightly longer but not statistically significant in patients treated with temsirolimus, while OS was significantly longer in those treated with sorafenib (12). The TIVO-1 trial showed that PFS was significantly longer in patients treated with tivozanib, while OS was slightly longer but not significantly in those treated with sorafenib (7). In the AXIS trial, the median PFS was significantly prolonged in the axitinib group compared with the sorafenib group, however, there was no significant difference in the median OS between groups (13). PFS may not be an appropriate surrogate endpoint for OS when comparing those drugs in this disease. The subsequent therapy which may affect improving OS should also be taken into consideration. Devising a way to use agents already approved, for example the cyclic treatment strategy were propounded (14), should be moved forward in order to improve survival of patients with metastatic RCC.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Cancer Research for the series “Renal Cell Carcinoma”. The article did not undergo external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2014.06.01). The series “Renal Cell Carcinoma” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hu-Lowe DD, Zou HY, Grazzini ML, et al. Nonclinical antiangiogenesis and antitumor activities of axitinib (AG-013736), an oral, potent, and selective inhibitor of vascular endothelial growth factor receptor tyrosine kinases 1, 2, 3. Clin Cancer Res 2008;14:7272-83. [PubMed]
- Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet 2011;378:1931-9. [PubMed]
- Hutson TE, Lesovoy V, Al-Shukri S, et al. Axitinib versus sorafenib as first-line therapy in patients with metastatic renal-cell carcinoma: a randomised open-label phase 3 trial. Lancet Oncol 2013;14:1287-94. [PubMed]
- Rixe O, Bukowski RM, Michaelson MD, et al. Axitinib treatment in patients with cytokine-refractory metastatic renal-cell cancer: a phase II study. Lancet Oncol 2007;8:975-84. [PubMed]
- Escudier B, Szczylik C, Hutson TE, et al. Randomized phase II trial of first-line treatment with sorafenib versus interferon Alfa-2a in patients with metastatic renal cell carcinoma. J Clin Oncol 2009;27:1280-9. [PubMed]
- Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 2007;356:125-34. [PubMed]
- Motzer RJ, Nosov D, Eisen T, et al. Tivozanib versus sorafenib as initial targeted therapy for patients with metastatic renal cell carcinoma: results from a phase III trial. J Clin Oncol 2013;31:3791-9. [PubMed]
- Mendel DB, Laird AD, Xin X, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res 2003;9:327-37. [PubMed]
- Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 2004;64:7099-109. [PubMed]
- Sonpavde G, Hutson TE, Rini BI. Axitinib for renal cell carcinoma. Expert Opin Investig Drugs 2008;17:741-8. [PubMed]
- Nakamura K, Taguchi E, Miura T, et al. KRN951, a highly potent inhibitor of vascular endothelial growth factor receptor tyrosine kinases, has antitumor activities and affects functional vascular properties. Cancer Res 2006;66:9134-42. [PubMed]
- Hutson TE, Escudier B, Esteban E, et al. Randomized phase III trial of temsirolimus versus sorafenib as second-line therapy after sunitinib in patients with metastatic renal cell carcinoma. J Clin Oncol 2014;32:760-7. [PubMed]
- Motzer RJ, Escudier B, Tomczak P, et al. Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol 2013;14:552-62. [PubMed]
- Nozawa M, Uemura H. Proposal of “cyclic therapy”, a novel treatment strategy with targeted agents for advanced renal cell carcinoma. Transl Androl Urol 2013;2:324-7.