
Diagnosis status and pathological diagnosis derived treatment of elderly lung cancer patients over 75 years old
Introduction
Lung cancer is usually more frequent in the elderly population and over two-thirds of new lung cancer cases in America are diagnosed in patients over 65 years old (1). With the aging tendency of population and increased lung cancer incidence, lung cancer in elderly population is becoming an increasingly important issue (2). Because of multiple chronic comorbidity and worries about complications related to invasive examinations, a disturbing number of elderly patients failed to receive pathological diagnosis and definitive anti-cancer therapy is thus delayed or unadopted.
Despite the significant improvement in lung cancer treatment, therapeutic outcome in elderly patients is usually worse than that in younger patients and complications are more frequent and severe (3). Remarkably, therapeutic in elderly lung cancer patients is conservative and elderly lung cancer patients are often undertreated with higher early death rates than younger patients (4). Meanwhile, elderly patients, especially those over 75 years old are more likely to be excluded by clinical trials (5). It appears that elderly patients did not benefit from the recent oncology advances as much as the young (6). All these current issues indicated the importance of evaluating the diagnosis and treatment in elderly lung cancer patients and we conducted this study to explore the diagnosis and treatment status of lung cancer in patients over 75 years old.
Methods
Patient selection
This study was performed at the department of respiratory and critical care medicine, Jinling Hospital (Nanjing, China) and was approved by Jingling Hospital’s Institutional Review Committee on Human Research. This retrospective study comprises 338 hospitalized patients diagnosed with lung cancer from September 1, 2010 to October 30, 2017. The inclusion criteria are as follows: (I) all included patients must be aged over 75 years; (II) patients must be diagnosed as primary non-small cell lung cancer (NSCLC) or small cell lung cancer (SCLC) by pathologic examination (histology and/or cytology examination) or clinical examination. Clinical diagnosis of lung cancer in this study must meet both of the following 2 diagnostic criteria: (I) typical imaging features of computed tomography (CT) or positron emission tomography/computed tomography (PET/CT); (II) at least one of the following increased blood tumor markers: carcinoembryonic antigen (CEA), cytokeratin 19 fragment (Cyfra21-1) or squamous cell carcinoma antigen (SCC). Any other patients who were suspicious of lung cancer and based solely on either imaging abnormalities or increased blood tumor markers were excluded to distinguish unreliable cases.
Clinical data collection
Patients’ demographic data at admission, main medical test results and treatment information were obtained through the electronic medical record system (EMR). Tumor staging was based on the 7th edition of TNM lung cancer staging system. Overall survival (OS) was defined as the length from the date of diagnosis or treatment initiation to the date of death by any cause or the last follow-up (June 21, 2018).
Statistical analysis
All statistical analyses were conducted by using PASW Statistics 18.0 (IBM Corporation, Armonk, NY, USA). Data are presented as mean ± SD or the absolute number of subjects. The test of normality and variance homogeneity was performed and Kruskal-Wallis test was applied if analysis of variance was not applicable. Survival curve was estimated by the Kaplan-Meier analysis and the log-rank test was utilized to examine the differences of survival between different groups. In all analyses, a P≤0.05 was considered to be statistically significant.
Results
Main patient characteristics
A total of 338 lung cancer inpatients were finally included in this study. The number of patients diagnosed as lung cancer in our institution added up to 2,791 during the same period. As shown in Table 1, 236 of the 338 patients were male and the mean age was 78.02 years old. The most frequent comorbidities were chronic obstructive pulmonary disease (COPD, n=126), pulmonary tuberculosis (PTB, n=45), diabetes mellitus (DM, n=39), hypertension (n=144), coronary heart disease (CHD, n=45), coronary stented (n=18), arrhythmia (n=34), chronic heart failure (CHF, n=12), cerebrovascular disease (CVD, n=34) and other malignant tumors (n=21); 42 of them were identified by routine medical examination or CT follow-up and all the other patients were hospitalized with symptoms including cough, sputum, hemoptysis, dyspnea, chest pain, fever, hoarseness, lymphadenectasis, weight loss and neurological symptoms.
Table 1
| Clinical features | Number |
|---|---|
| Male/female | 256/82 |
| Age, years | 78.02±2.94 |
| Smoking | |
| Non-smokers | 135 |
| Smokers | 203 |
| Co-morbidities | |
| COPD | 126 |
| PTB | 45 |
| DM | 39 |
| Hypertension | 144 |
| CHD | 45 |
| Coronary stented | 18 |
| Arrhythmia | 34 |
| CHF | 12 |
| CVD | 34 |
| Other types of cancer | 21 |
| Performance status | |
| 0–1 | 252 |
| 2 or more | 86 |
| Main symptoms | |
| Cough | 96 |
| Sputum | 138 |
| Hemoptysis | 72 |
| Dyspnea | 100 |
| Chest pain | 51 |
| Fever | 24 |
| Hoarseness | 9 |
| Lymphadenectasis | 8 |
| Weight loss | 7 |
| Neurological symptoms | 6 |
| Medical examination only | 42 |
| Initial stage of NSCLC | |
| I | 19 |
| II | 22 |
| III | 67 |
| IV | 191 |
| Initial stage of SCLC | |
| Limited disease | 10 |
| Extensive disease | 29 |
COPD, chronic obstructive pulmonary disease; PTB, pulmonary tuberculosis; DM, diabetes mellitus; CHD, coronary heart disease; CHF, chronic heart failure; CVD, cerebrovascular disease.
Diagnosis and treatment results
Among all the 338 patients diagnosed with lung cancer, 290 (85.80%) got pathological diagnosis while the other 48 patients (14.20%) were clinically diagnosed (Table 2). As stated above, clinically diagnosis was based on both typical imagological examination and elevated blood tumor markers.
Table 2
| Diagnosis information | Number |
|---|---|
| Diagnostic mode | |
| Clinical diagnosis | 48 |
| Pathological diagnosis | 290 |
| Invasive examination | |
| TBNA/TBLB | 147 |
| EBUS-TBNA | 8 |
| CT-PTNB | 159 |
| Closed thoracic drainage | 68 |
| Pleural biopsy | 23 |
| Superficial lymph node biopsy | 8 |
| Complications related to invasive examination | |
| Hemoptysis | 45 |
| Pneumothorax | 20 |
| Dyspnea | 2 |
| Complications with treatment | 37 |
| Reasons for failure of pathological diagnosis | |
| Negative pathological diagnosis | 11 |
| Patients and relatives unwillingness | 32 |
| Unable to tolerate | 5 |
| Types of pathology | |
| AC | 132 |
| SCC | 96 |
| SCLC | 38 |
| Undifferentiated | 17 |
| Mixed | 3 |
| Other | 4 |
| EGFR status | |
| Wild | 84 |
| Exon 19 del | 26 |
| Exon 21 L858R | 15 |
| Exon 21 L858R & T790M | 1 |
| Unknown | 212 |
| ALK status | |
| Negative | 122 |
| Positive | 2 |
| Unknown | 214 |
TBLB, transbronchial lung biopsy; EBUS-TBNA, endobronchial ultrasound-guided transbronchial needle aspiration; CT-PTNB, computed tomography-guided percutaneous transthoracic needle biopsy; AC, adenocarcinoma; SCC, squamous carcinoma; SCLC, small cell lung cancer.
Invasive examination taken in this study included conventional transbronchial needle aspiration (TBNA)/transbronchial lung biopsy (TBLB), endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA), CT-guided percutaneous transthoracic needle biopsy (CT-PTNB), closed thoracic drainage, pleural biopsy and superficial lymph node biopsy. Main complications related to invasive examination were hemoptysis (n=45), pneumothorax (n=20) and dyspnea (n=2) and 37 of them need further treatment. None of these complications were potentially-crippling or fatal. Among the 48 clinically diagnosed patients, 11 also received invasive examination but failed to get a definite pathological diagnosis. Most of all, 32 patients did not receive invasive examination because of their own and relatives unwillingness without any contraindications.
Among the pathological diagnosis, adenocarcinoma (132/290), squamous carcinoma (96/290) and small cell lung cancer (38/290) were the most frequent pathological type. Most patients were staged III (n=67) or IV (n=191) at the time of initial diagnosis. Epidermal growth factor receptor (EGFR) detection was performed in 126 patients and 42 patients were mutated. Exon 19 deletion was detected in 26 patients and L858R was detected in 15 patients. One patient was primarily doubly mutated with L858R and T790M. AKL-EML4 fusion gene were detected in 124 patients and only 2 of them were positive. All these molecular examinations utilized tissue specimens or cell blocks.
Twenty-four early stage patients were treated by surgery. Five of them received wedge resection and the other 19 patients received lobectomy and lymph node dissection (Table 3). Radiation therapy was used in 50 patients. Cyberknife and 3D-conformal radiotherapy were mostly used. Radiotherapy regions included pulmonary and/or hilar lymph node, brain and bones. Four patients received radiotherapy of both the primary focus and the metastasis. Systemic therapeutics were conducted in 188 patients. Main treatment included chemotherapy (n=128), target treatment (n=60). None of these patients ever participate in any clinical trials.
Table 3
| Treatment | Number |
|---|---|
| Initial radiotherapy | |
| Pulmonary | 34 |
| Brain | 7 |
| Bone | 5 |
| Multiple sites | 4 |
| Radiotherapy regimen | |
| Cyberknife | 21 |
| Conformal radiotherapy | 24 |
| Whole brain radiotherapy | 3 |
| Multiple | 2 |
| Surgery | |
| Wedge resection | 5 |
| Lobectomy | 19 |
| Chemotherapy | |
| Single-agent | 43 |
| Double-agent | 85 |
| Target therapy | |
| EGFR-TKIs | 59 |
| ALK-TKI | 1 |
EGFR-TKI, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor; ALK, anaplastic lymphoma kinase.
Survival analysis
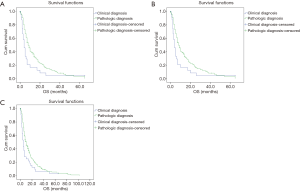
We firstly analyze the survival of clinically diagnosed patients and pathologically diagnosed patients receiving best supportive treatment (BST). As shown in Figure 1, the OS of patients in these two groups was extremely similar in both stage I–III (p=0.760) and stage IV (P=0.579) patients. On the other hand, these results indicated that the clinical diagnosis of lung cancer in this study is reliable.

As stated above, the clinical diagnosis and staging were reliable. Base on this, we conducted Kaplan-Meier survival analysis based on diagnosis method in different stages. As shown in Figure 2, no significant difference was detected in stage I–III (P=0.393) patients. In stage IV patients, OS of pathologically diagnosed patients were significantly longer than clinically diagnosed patients (median OS: 8 vs. 4 months, P=0.027).
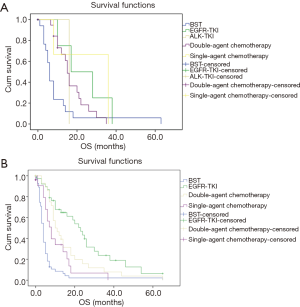
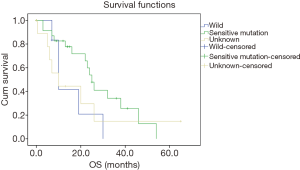
Then we further analyzed the effect of different systemic therapeutics on OS in stage III and IV patients. As shown in Figure 3A, aggressive treatment showed a tendency to improve OS than BST in stage III patients with no significant difference (P=0.06). In stage IV patients (Figure 3B), more aggressive treatment of chemotherapy and target treatment got much longer than BST (EGFR-TKIs vs. double agent treatment vs. single agent treatment vs. BST: 23 vs. 10 vs. 8 vs. 4 months, EGFR-TKIs vs. BST: 23 month vs. 4, P<0.001, double agent chemotherapy vs. BST: P<0.001, single agent chemotherapy vs. BST: P=0.006). No significant difference was detected between double agent chemotherapy and single agent chemotherapy (P=0.122). Since most patients treated by EGFR-TKIs were stage IV (n=49), we analyzed the EGFR status on survival in stage IV patients. As shown in Figure 4, the OS was much longer in sensitive EGFR-mutated patients (sensitive mutation vs. wild vs. unknown: 23 vs. 10 vs. 10 months, P=0.039) and no significant difference was detected between EGFR-wild and EGFR-unknown patients (P=0.948).
Discussion
In this study, it is demonstrated that about 14% of hospitalized elderly lung cancer patients aged over 75 years were not diagnosed pathologically and 9.5% of the inpatients reject invasive examination with no contraindication. Complications of invasive examination were acceptable and remediable. The survival of patients who could not get a pathological diagnosis or molecular examination was significantly worse than pathologically diagnosed patients. These results evoke the implementation of pathological diagnosis, molecular examination and anti-cancer treatment in very elderly lung cancer patients.
Elderly patients over 70 years account about 40% of lung cancer patients (7). It is widely accepted that elderly patients are accompanied with higher incidence of chronic diseases and survival of elderly lung cancer patients is more dissatisfactory (8). Eberle et al. conducted a study covering around one third of the German population and included 132,612 lung cancer patients diagnosed between 2002 and 2010. It was indicated that the 5-year relative survival rate in lung cancer patients over 80 years old was less than half of that in patients below 60 years old (8.4% vs. 18.5% in men and 10.6% vs. 23.7%) (9). This might be due to worse physical condition, less willing for definite diagnosis, lower therapeutic usage and higher rate of severe adverse events (4,10,11). Our results show that survival of elderly patients with a definite pathological diagnosis is much better than clinically diagnosed patients in advanced stages. Survival analysis also indicates that the OS of clinically diagnosed patients is equal to that of pathologically diagnosed patients treated with BST only. This indicates that clinical diagnosis based on imaging examination and tumor markers is reliable and elderly patients could also benefit from a confirmed diagnosis. It is quite important to take pathological diagnosis in these elderly patients highly suspected of lung cancer.
Previous studies have partially demonstrated the effectiveness and safety of conventional minimally invasive examinations in elderly lung cancer patients. It is revealed that conventional TBNA (12) and EBUS-TBNA (13) are valuable, safe and well tolerated procedure in patients over 70 years old. A recent study showed that the mean age of complication-present patients was slightly beyond than that of complication-absent patients with no significant statistical difference (69.4±11.5 vs. 66.3±11.9) (14). Despite all this, the rate of pathological diagnosis of lung cancer is relatively lower in elderly patients. Innos and his colleges reviewed the Estonian Cancer Registry data through 1995 to 2008 and founded that only 51.6% lung cancer patients aged between 75 and 84 years old were microscopically verified compared with 86.2% in patients aged below 55 years old (15). Our study indicated that even among inpatients, over 14% patients do not get the pathological diagnosis. Taken outpatients, substrate hospitals and economic conditions in consideration, the average rate of pathological diagnosis should be substantially decreased across China. Patients receiving invasive diagnosis in this present study were common and validated including conventional TBNA, TBLB, EBUS-TBNA, CT-PTNB, closed thoracic drainage, pleural biopsy and superficial lymph node biopsy. The most frequent complication is hemoptysis followed by pneumothorax and dyspnea. Notably, none fatal complications occurred. These results indicated that these minimally invasive examinations were safe in elderly patients. Recent advance in liquid biopsy technologies including circulating tumor cells, nucleic acid and exosome are potent supplementary examinations to conventional invasive examination, especially in advanced lung cancer patients (16). This current study also demonstrates the value of EGFR detection in survival improvement.
Despite the fact that a large proportion of patients with lung cancer are elderly, information remains scant on how best to treat these patients. For early stage elderly patients, SBRT (stereotactic body radiotherapy) and surgery has been regarded as primary therapeutic options. For operably recurrent patients aged over 75 years surgically resection could also achieved satisfactory long-term outcomes (17). It was demonstrated that SBRT is well tolerated and feasible in stage I patients even older than 85 years old (18). Our present study indicated that 24 of 338 patients received surgery with no severe complications. Fifty patients with different stages received radiotherapy which is feasible and tolerant.
Multiple studies have indicated that elderly patients are less willing to adopt systemic treatment, especially chemotherapy (4,11,19). It was shown that elderly patients older than 65 years old could benefit from adjuvant chemotherapy with increment of 5-year OS varying between 4.1% and 15% (20). For patients over 70 years old with a PS scores of 0–2, platinum-based doublet chemotherapy was associated with survival benefits compared with vinorelbine/gemcitabine monotherapy by a phase III randomised trial (21). Similar results were also found in a high-quality meta-analysis (22). As for anti-angiogenic therapy, a recent secondary analysis of the ECOG 4599 and PointBreak trials demonstrated that patients between 65 and 75 years benefit from the addition of bevacizumab to paclitaxel-carboplatin chemotherapy. However, no benefit was observed in patients aged over 75 years. Target therapeutic in elderly patients were more acceptable because of relatively slighter adverse effects. Besides the first generation of EGFR-TKIs, afatinib was shown to be able to improve OS versus chemotherapy in elderly patients aged 75 years or more with sensitive mutations (23). A phase II study focusing on the treatment of second or third line erlotinib in unselected patients over 75 years old found that even though about one third patients required a dose reduction, erlotinib is still a useful therapeutic option (24). These studies revealed the survival benefit of slightly aggressive systemic treatment with tolerable adverse effects. But in consideration of the actuality that most studies are inclined to exclude patients with major comorbidities, its effect in clinical determination is restricted. This current study also revealed that systemic therapeutics were associated with survival benefit than BST and clinical diagnosis with no anti-cancer treatment. Double agent chemotherapy is tended to be superior to single agent chemotherapy with no statistical difference. EGFR-TKIs were more effective in patients with sensitive mutations with a median OS of 23 months. Unrestricted first line EGFR-TKIs in patients with none EGFR examinations benefit little. Taken the medical products donating projects of different EGFR-TKIs in consideration, neither survival nor economic benefit were obtained. These results indicated the importance of pathological and molecular examination and slightly aggressive treatment in selected elderly patients.
Worth the whistle, older patients are generally clinical trial non-participants although they constitute the majority of advanced lung cancer patients. Sacher reviewed all the 248 phase III trials of systemic therapy for advanced NSCLC between 1980 and 2010 in 2013. It was demonstrated that 33 of the 100 most cited trials specifically excluded elderly patients in their trial design (age exclusion ranged from >65 to >75 years of age) (25). One main reason for this might be the case that most of these trials were chemotherapy involved. None of the patients included in this study ever participate in any clinical trial. Taking account of the fact that recent trials of new target therapeutic drugs and immunotherapy mostly did not specially exclude elderly patients, sponsor’s attitude to elderly patients is getting favourable.
This is a retrospective single-center study and only inpatients were involved. Nevertheless, this is the first study investigating the effect of pathological diagnosis on survival in Chinese elderly patients over 75 years old. Future larger-scaled multicenter trials and prospective studies focusing on elderly patients is needed to verify this.
Taken all together, it was revealed that pathological diagnosis of elderly lung cancer patients over 75 years old is insufficient in China. Pathological diagnosis and aggressive treatment are associated with improved survival. More efforts should be made to encourage elderly patients to accept invasive examination and receive pathological diagnosis. Future well-designed, prospective studies are in need to verify this conclusion.
Acknowledgments
Funding: This present study was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.12.35). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Jingling Hospital’s Institutional Review Committee on Human Research (IRB number: 2018NZGKJ-061) and as this is a retrospective study, the informed consent of patients was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Barta JA, Zinner RG, Unger M. Lung Cancer in the Older Patient. Clin Geriatr Med 2017;33:563-77. [Crossref] [PubMed]
- Zhang X, Wu L, Xu Y, et al. Trends in the incidence rate of lung cancer by histological type and gender in Sichuan, China, 1995-2015: A single-center retrospective study. Thorac Cancer 2018;9:532-41. [Crossref] [PubMed]
- Losanno T, Gridelli C. Recent advances in targeted advanced lung cancer therapy in the elderly. Expert Rev Anticancer Ther 2017;17:787-97. [Crossref] [PubMed]
- Costa GJ, de Mello MJG, Ferreira CG, et al. Undertreatment trend in elderly lung cancer patients in Brazil. J Cancer Res Clin Oncol 2017;143:1469-75. [Crossref] [PubMed]
- Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA 2004;291:2720-6. [Crossref] [PubMed]
- Zeng C, Wen W, Morgans AK, et al. Disparities by Race, Age, and Sex in the Improvement of Survival for Major Cancers. JAMA Oncology 2015;1:88. [Crossref] [PubMed]
- Gridelli C, Maione P, Rossi A, et al. Management of unfit older patients with advanced NSCLC. Cancer Treatment Reviews 2009;35:517-21. [Crossref] [PubMed]
- Lembicz M, Gabryel P, Brajer-Luftmann B, et al. Comorbidities with non-small cell lung cancer: Is there an interdisciplinary consensus needed to qualify patients for surgical treatment? Ann Thorac Med 2018;13:101-7. [Crossref] [PubMed]
- Eberle A, Jansen L, Castro F, et al. Lung cancer survival in Germany: A population-based analysis of 132,612 lung cancer patients. Lung Cancer 2015;90:528-33. [Crossref] [PubMed]
- Borghaei H, Yim YM, Guerin A, et al. Severe adverse events impact overall survival and costs in elderly patients with advanced non-small cell lung cancer on second-line therapy. Lung Cancer 2018;119:112-9. [Crossref] [PubMed]
- Wang S, Wong ML, Hamilton N, et al. Impact of Age and Comorbidity on Non–Small-Cell Lung Cancer Treatment in Older Veterans. J Clin Oncol 2012;30:1447-55. [Crossref] [PubMed]
- Vitale C, Galderisi A, Maglio A, et al. Diagnostic yield and safety of C-TBNA in elderly patients with lung cancer. Open Med (Wars) 2016;11:477-81. [Crossref] [PubMed]
- Evison M, Crosbie PA, Martin J, et al. EBUS-TBNA in elderly patients with lung cancer: safety and performance outcomes. J Thorac Oncol 2014;9:370-6. [Crossref] [PubMed]
- Iezzi R, Larici A, Contegiacomo A, et al. A new score predicting intraprocedural risk in patients undergoing CT-guided percutaneous needle pulmonary biopsy (CATH-score). Eur Rev Med Pharmacol Sci 2017;21:3554-62. [PubMed]
- Innos K, Lang K, Parna K, et al. Age-specific cancer survival in Estonia: recent trends and data quality. Clin Epidemiol 2015;7:355-62. [Crossref] [PubMed]
- Santarpia M, Liguori A, D'Aveni A, et al. Liquid biopsy for lung cancer early detection. J Thorac Dis 2018;10:S882-97. [Crossref] [PubMed]
- Takenaka T, Inamasu E, Yoshida T, et al. Post-recurrence survival of elderly patients 75 years of age or older with surgically resected non-small cell lung cancer. Surg Today 2016;46:430-6. [Crossref] [PubMed]
- Hayashi S, Tanaka H, Kajiura Y, et al. Stereotactic body radiotherapy for very elderly patients (age, greater than or equal to 85 years) with stage I non-small cell lung cancer. Radiat Oncol 2014;9:138. [Crossref] [PubMed]
- Veluswamy RR, Levy B, Wisnivesky JP. Chemotherapy in elderly patients with nonsmall cell lung cancer. Curr Opin Pulm Med 2016;22:336-43. [Crossref] [PubMed]
- Poudel A, Sinha S, Gajra A. Navigating the Challenges of Adjuvant Chemotherapy in Older Patients with Early-Stage Non-Small-Cell Lung Cancer. Drugs Aging 2016;33:223-32. [Crossref] [PubMed]
- Quoix E, Zalcman G, Oster JP, et al. Carboplatin and weekly paclitaxel doublet chemotherapy compared with monotherapy in elderly patients with advanced non-small-cell lung cancer: IFCT-0501 randomised, phase 3 trial. Lancet 2011;378:1079-88. [Crossref] [PubMed]
- Santos FN, de Castria TB, Cruz MR, et al. Chemotherapy for advanced non-small cell lung cancer in the elderly population. Cochrane Database Syst Rev 2015;CD010463. [PubMed]
- Wu YL, Sequist LV, Tan EH, et al. Afatinib as First-line Treatment of Older Patients With EGFR Mutation-Positive Non–Small-Cell Lung Cancer: Subgroup Analyses of the LUX-Lung 3, LUX-Lung 6, and LUX-Lung 7 Trials. Clin Lung Cancer 2018;19:e465-79. [Crossref] [PubMed]
- Yamada K, Azuma K, Takeshita M, et al. Phase II Trial of Erlotinib in Elderly Patients with Previously Treated Non-small Cell Lung Cancer: Results of the Lung Oncology Group in Kyushu (LOGiK-0802). Anticancer Res 2016;36:2881-7. [PubMed]
- Sacher AG, Le LW, Leighl NB, et al. Elderly patients with advanced NSCLC in phase III clinical trials: are the elderly excluded from practice-changing trials in advanced NSCLC? J Thorac Oncol 2013;8:366-8. [Crossref] [PubMed]


