
MT-12 inhibits the growth and metastasis of bladder cancer cells via suppressing tumor angiogenesis in vivo and in vitro
Introduction
Bladder cancer (BC) is the most prevalent genitourinary cancer and ranks as the ninth leading cause of cancer-related death in the world (1). Recurrence and metastasis are two major features of BC. In particularly, metastasis is the principal cause of BC mortality. Although many BC drugs have been reported, it is still necessary to discover new drugs to treat BC.
Cobra venom membrane toxin (MT), also known as cardiotoxin (CTX) or cytotoxin (CT), has been defined as a major subset of cobra venom having cardiac toxicity and anticancer activity properties (2). Several recent studies reported that MT causes a volume reduction in different types of tumors (like hepatoma and prostate cancer) in vivo, prolongs the survival of tumor-bearing mice, and inhibits tumor metastasis (3,4). The biological effects of MT may be caused by damaging the membrane structure of tumor cells, interfering with DNA synthesis, and inducing apoptosis (5).
Chinese cobra venom membrane toxin (MT-12), which is isolated from the snake venom of the Chinese Naja naja atra, has been found to exhibit a variety of bioactivities with anticancer potential in many types of cancer cells (6). In our previous study, increasing concentrations of MT-12 were preliminarily proven to decrease the invasion ability of the BC cell line, EJ, without causing damage to the normal bladder epithelium (7). However, the mechanism by which MT-12 suppresses BC cells remains unclear, and the inhibitory effect has not been confirmed in other bladder cell lines. In this study, we investigated the effect of MT-12 on the proliferation, adhesion, invasion, and metastasis of the BC cell lines, RT4 and T24, and explored its possible mechanism.
Tumour angiogenesis is an essential process that drives tumor growth beyond a diffusion limit size and enhances metastasis, which can facilitate an increased blood supply and increased the availability of growth factors (8). Additionally, angiogenesis is assumed to require the degradation of different components of the matrix, which allows tumor cells to pass the basal membrane and enter the lymphatic systems and peripheral blood. Matrix metalloproteinases (MMPs) are responsible for the degradation of some extracellular matrix (ECM) components (9). MMP-2 and MMP-9 have been proven to associate with tumor dissemination and invasiveness strongly, and they degrade type IV collagen and gelatine substrates (10). Furthermore, many studies have indicated that vascular endothelial growth factor (VEGF) is a multifunctional cytokine that plays a pivotal role in the regulation of angiogenesis (11), which can promote blood vessels and lymphatic hyperplasia (12). Also, many cell adhesion molecules (CAMs), such as vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1), have been found to play pivotal roles in the adhesion of cancer cells to the vascular endothelium, and this process is thought to be important for the angiogenic process (13) and dissemination of tumour cells to new sites (14,15). Therefore, targeting tumor angiogenesis may be a promising therapeutic strategy for BC. Therefore, in our study, the expression of MMP-2, MMP-9, VEGF, VCAM-1 and ICAM-1 in MT-12-treated BC cells was investigated to determine the potential molecular mechanism of MT-12 treatment of BC.
Methods
Materials
MT-12, which was purified from the venom of the Chinese Naja naja atra, was obtained from the Laboratory of Animal Toxins, Kunming Institute of Zoology, Chinese Academy of Medical Sciences, China, as a gift. The MT-12 was dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at a stock solution of 4 mM and stored at −20 °C. The human BC cell lines (T24 and RT4) were obtained from our institute (Yunnan Institute of Urology). A total of 32 BALB/c nude mice (age, 5–8 weeks, 18–20 g) were purchased from the Animal Center of Sun Yat-sen University (Guangzhou, China).
Cell culture
Human BC cell lines (T24 and RT4) were cultured in RPMI-1640 (Gibco; Thermo Fisher Scientific Inc., Waltham, MA, USA). Cells supplemented with 10% fetal bovine serum (FBS; HyClone, Logan, UT, USA) were grown in 25 cm2 cell culture flasks (Corning Inc., Corning, NY, USA) at 37 °C with 5% CO2.
Cell proliferation assay (CCK8)
Cells were added into a 96-well plate at 100 µL/well (2×104 cells) and pre-cultured with RPMI-1640 for 24 h. According to previous results, the IC50 of MT-12 in BC cells was approximately 0.51–0.66 µg/mL (7); RT4 and T24 were treated with MT-12 (0.1, 0.25 and 0.5 µg/mL) for 24 h. Cell proliferation was determined using a CCK-8 assay (10 µL/well). An inverted microscope (OLYMPUS, Japan) was used to observe the changes in cell morphology in each treatment group. Absorbance was measured at 490 nm after 1 h.
Detection of cell apoptosis
The Annexin V-FITC/PI kit (Dojindo, China) was used for detecting cell apoptosis according to the manufacturer’s instructions. Briefly, 1× Annexin V Binding Solution was pre-mixed in a cell-seeded plate to make a cell suspension with a final concentration of 1×106 cells/mL. The cells were washed 3 times with phosphate-buffered saline (PBS) and then mixed with 100 µL Annexin V binding buffer, followed by the addition of 5 µL each of Annexin V and PI. After incubation for 10–15 min in the dark, Annexin V binding buffer was added to bring the total volume to 1 mL. The cells were then transferred into a flow cytometry tube and analyzed using a flow cytometer (Ex =488 nm, Em =530 nm).
Wound-healing assay
The migration of cells was examined by a wound-healing assay. T24 and RT4 cells were seeded in a 6-well plate at 5×105 cells/well and incubated at 37 °C until they reached 90–100% confluency. Confluent cells were scratched with a pipette tip (200 µL) perpendicular to the back of the well. Then, the cells were treated with vehicle (PBS), 0.25 or 0.5 µg/mL MT-12. After 24 h of culture at 37 °C in 5% CO2, cell migration onto the wound surface was determined under an inverted microscope (OLYMPUS, Japan).
Cell adhesion experiment
A total of 100 µL (2×104 cells/mL) of cells were added in each well of a 96-well plate and then incubated at 37 °C in 5% CO2 for 3 h. Then, the cells were treated with vehicle (PBS), 0.25 or 0.5 µg/mL MT-12. After incubation for 24 h, the cell suspension was removed, each well was washed with PBS 3 times, 0.5% crystal violet was added at room temperature for 10 min, and the cells were observed under a light microscope (×20 objective). For quantification, the adhered cells on the wells were extracted with 33% acetic acid. The absorbance of the eluted stain was determined at 570 nm.
Quantitative real-time PCR analysis
Total RNA was extracted using TRIzol reagent (Invitrogen). A total of 2 µg RNA was reverse transcribed into cDNA using a ReverTra Ace qPCR RT Kit (Toyobo Life Science, Osaka, Japan), according to the manufacturer’s protocol. The reverse transcription steps were as follows: 37 °C for 15 min and 98 °C for 5 min. The samples were stored at −20 °C. qPCR was performed using an SYBR® Green Real-Time PCR Master Mix (Toyobo Life Science) and an ABI StepOnePlus™ Real-Time PCR System (ABI; Thermo Fisher Scientific Inc.) according to the manufacturers’ protocols. GAPDH was used as an endogenous control. The PCR thermocycling conditions were as follows: initial denaturation at 95 °C for 3 min followed by 40 cycles of 95 °C for 15 s and extension at 60 °C for 1 min. The detailed sequences of primers used are shown below: ICAM-1 sense, 5'-ACCACAGGAGCAACTTCT-3'; antisense, 5'-CGTTCAGGACCACTTCAC-3'; VCAM-1 sense, 5'-ACTTCTGGTTGCTCTATTGTG-3'; antisense, 5'-CAGTCATCTCAGTGGTAGTG-3'; VEGF sense, 5'-AGGGCAGAATCATCACGAA-3'; antisense, 5'-TCTTGCTCTATCTTTCTTTGGTCT-3'; β-actin sense, 5'-TCCGTGACATCAAGGAGAAGC-3'; antisense, 5'-GCACCGTGTTGGCGTAGAG-3'. The final data were analyzed by the 2−△△Ct method. Each experiment was repeated 3 times.
Gel zymography assay
The enzymatic activity of MMP-9 and MMP-2 was determined by gelatin zymography, and conditioned media were briefly prepared with standard SDS-gel loading buffer containing 0.01% SDS without β-mercaptoethanol and without boiling before loading. Then, prepared samples were subjected to electrophoresis with 8% SDS polyacrylamide gels containing 0.5% gelatin. After electrophoresis, gels were washed twice with 50 mL distilled water containing 2% Triton X-100 for 30 min at room temperature to remove SDS and then incubated in 50 mL reaction buffer (40 mm Tris-HCl, pH 8.0, 10 mm CaCl2, 0.02% NaN3) for 24 h at 37 °C, stained with Coomassie brilliant blue R-250 and destained with destaining solution (20% methanol, 10% acetic acid, and 70% water).
ELISA
After 24 h of incubation, the cell culture supernatants were harvested and stored at –20 °C until quantification of VEGF by enzyme-linked immunosorbent assay (ELISA). The assays were performed according to the providers’ protocols.
Nude mice subcutaneous tumor formation experiment
The nude mice were maintained in sterile conditions, following a protocol approved by Kunming Medical University’s Institutional Animal Care and Use Committee, and the institutional and national guide for the care and use of laboratory animals was followed. After disinfecting the skin, cultured RT4 cells from each group were suspended and subcutaneously injected into the left subaxillary of each nude mouse. Groups were set as follows: RT4, RT4 + 0.1 µg/kg MT-12, RT4 + 0.25 µg/kg MT-12, and RT4 + 0.5 µg/kg MT-12. The nude mice were fed in specific pathogen free (SPF) level. Four weeks later, the mice were euthanized, and the tumor volume was measured to make a tumor growth curve. Tumour volume (mm3) = m12×m2×0.5236 (m1, the shortest tumour diameter; m2, the longest tumour diameter).
Immunohistochemistry
Formalin-fixed tissue specimens were embedded in paraffin, and the paraffin-embedded samples were then cut into serial sections (4 µm thick). Partial tumors were stained with hematoxylin and eosin (H&E), and tumor specimens were immunostained with cleaved caspase 3, VEGF and ICAM-1. Images were captured under a light microscope (Olympus, Japan). Positive cells were calculated under 200× magnification.
Statistical analysis
Statistical analyses were performed by SPSS 19.0. Data were presented as the mean ± standard deviation. ANOVA was used for group count data statistical analysis. P<0.05 was considered to indicate statistical significance.
Results
MT-12 inhibits the proliferation, migration, and adhesion of BC cell lines
To examine the effect of MT-12 on BC cell proliferation, we employed CCK-8 assays to characterize RT4 and T24 cell line proliferation in response to MT-12. As shown in Figure 1A, 0.1 µg/mL MT-12 had no significant effect on the proliferation of both cell lines, while the proliferation of RT4 and T24 cells were significantly inhibited by 0.25 and 0.5 µg/mL MT-12. Subsequently, the promoting effects of 0.5 µg/mL MT-12 on apoptosis were examined. The results showed that 0.5 µg/mL MT-12 could significantly promote the apoptosis of RT4 and T24 cells over time (Figure 1B).
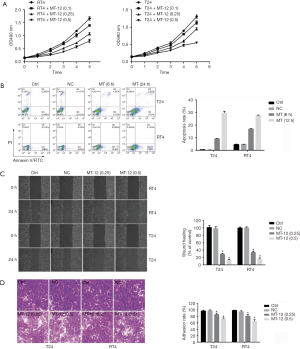
Furthermore, we assessed the effect of MT-12 on the migration of BC cells. Compared with vehicle treatment, the wound-healing assay showed that 0.25 and 0.5 µg/mL MT-12 could significantly inhibit the migration of RT4 and T24 cells into the wounded area in a concentration-dependent manner (Figure 1C).
After this, another property associated with metastasis-adherence was detected. The experimental results showed that the adhesion of BC cells could be inhibited by 0.25 or 0.5 µg/mL MT-12 compared with the control treatment (Figure 1D).
MT-12 inhibits the invasion and metastasis of BC through inhibiting tumor angiogenesis in vitro
The overexpression of MMPs is a critical step for tumor angiogenesis and metastasis, and ECM metalloproteinases lead to a poor prognosis of cancer. We wanted to determine whether the effect of MT-12 inhibiting BC migration involves the regulation of the expression of MMPs. The expression of MMP-2 and MMP-9 were detected by gel zymography assays. The results showed that MT-12 treatment upregulated the expression of MMP-9 in T24 or RT4 cell lines but had no significant effect on MMP-2 in T24 or RT4 cell lines. When we used PMA (a tumor accelerator) as a pretreatment, MT-12 effectively inhibited the PMA-induced MMP-9 upregulation, but no significant effect on the level of MMP-2 was observed (Figure 2A).
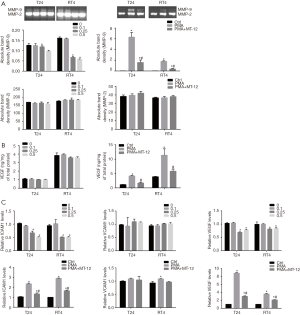
The expression of VEGF also plays a key role in regulating the progression of BC. We examined the expression of VEGF in BC cells after treatment with MT-12. ELISA results showed that different concentrations of MT-12 did not change the expression of VEGF. However, after PMA upregulated the expression of VEGF in BC cells, MT-12 inhibited the related VEGF expression induced by PMA (Figure 2B).
ICAM-1 and VCAM-1 are also crucial for tumor angiogenesis and adhesion. We detected the mRNA levels of ICAM-1, VCAM-1, and VEGF in bladder cancer cells after MT-12 treatment by RT-PCR. The RT-PCR result showed that, with the increase in MT-12 concentration, the levels of ICAM-1 and VEGF mRNA were significantly downregulated, while the level of VCAM-1 did not change. Moreover, MT-12 inhibited the upregulation of ICAM-1 and VEGF mRNA induced by PMA in T24 and RT4 cell lines and had no effect on the expression of VCAM-1 (Figure 2C).
MT-12 inhibits tumor growth in vivo
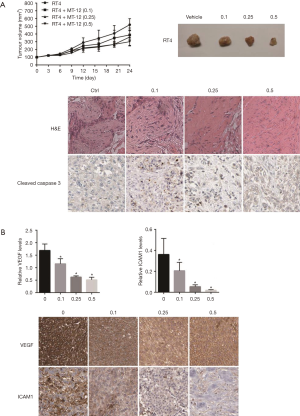
The antitumor effects of MT-12 in vivo were examined using a subcutaneously transplanted tumor model in nude mice. The results showed that MT-12 could inhibit the tumor growth of subcutaneously implanted RT4 cells in nude mice in a concentration-dependent manner (Figure 3A). To clarify whether the effect by which MT-12 inhibited BC cells correlated with the expression of VEGF and ICAM-1, the expression of VEGF and ICAM-1 in the tumor tissue was detected by immunohistochemistry. Fluorescence quantitative results showed that MT-12 could effectively inhibit the expression of VEGF and ICAM-1 in a concentration-dependent manner (Figure 3B).
Discussion
This study was performed to elucidate the role of MT-12 in the proliferation, apoptosis, migration, and adhesion of BC cells in vivo and vitro. We also explored the underlying mechanism.
MT, an active component of the venom from the Naja naja atra, is considered a major potential anticancer property of snake venom (16). MT can inhibit tumor functions through diverse modes, such as breaking the cytomembrane and activating necrotic or apoptotic cell death pathways in cancer cells (17). Many studies have examined the toxicity of MT in several of the cancer cell lines (MCF-7, P388, K562, and H22) and normal human cell lines (16HBE); the order of cytotoxicity was MCF-7> P388≈K562> H22≈16HBE, indicating that MT is relatively selective for certain cancer cell types. Moreover, this study found that MT can be significantly and selectively functional in anticancer activity by promoting programmed cell death through the mitochondrial and/or lysosomal pathways (18).
MT-12, which is isolated from the Chinese Naja naja atra, has been proven to inhibit cancer cells (6) selectively. For BC, our previous study showed that MT-12 could effectively inhibit the growth of the highly invasive BC cell line EJ in a concentration-dependent manner; the IC50 of EJ treated with MT-12 for 72 h was 0.66 µg/mL (7). Incidentally, our studies showed that the invasion ability of the BC cell line EJ was reduced with increasing MT-12 concentration but without obvious damage to the normal urinary tract epithelial cells (7,19).
In this study, we proved that MT-12 could inhibit the proliferation, migration, and adhesion of BC cells and promote the apoptosis of BC cells and that this antitumor effect is concentration-dependent in RT4 and T24 cell lines. Furthermore, in vivo, MT-12 can inhibit the growth of subcutaneous RT4 cells in nude mice in a concentration-dependent manner. These results were consistent with our previous findings.
Furthermore, we investigated whether inhibiting tumor angiogenesis is a possible mechanism for the above effects. Angiogenesis is essential for the progression of many types of tumors because the growth of tumors depends on vascular nutrition (20). MMPs may play a vital role in tumor angiogenesis and metastasis by degrading ECM components. MMPs can open gaps between endothelial cells, and then, their secretory products, TGFb1 and VEGF, can promote endothelial cell contraction and accelerate the invasion of a tumor to the blood vessels (21,22). VEGF is also a potent angiogenic factor for blood vessel formation to regulate the progression of tumors (23,24), it can bind to its receptor, such as VEGFR1, activate the PI3K/AKT signal, and then promote the proliferation and angiogenesis in tumors (25). The expression of MMP-2/9, VEGF, and other factors may play a key role in regulating the progression of BC (26). In our study, 0.25 and 0.5 µg/mL of MT-12 significantly inhibited the expression of MMP-9 in BC cell lines, but the expression of VEGF did not change in the MT-12-treated BC cell lines. However, RT-PCR results showed that the level of VEGF mRNA in BC cells was significantly downregulated after treatment with MT-12, and MT-12 effectively inhibited the expression of VEGF in a transplanted tumor model in nude mice. The reason for this phenomenon might relate to the post-transcriptional regulation or inhibition of VEGF degradation after MT-12 treatment, but this mechanism needs further exploration. When we used PMA (a tumor accelerator) as a pre-treatment, MT-12 could effectively inhibit the PMA-induced MMP-9 and VEGF upregulation. According to these results, our study indicated that the inhibitory effect of MT-12 on BC cells might be related to regulating MMP-9 and VEGF expression, which are essential for tumor angiogenesis.
Moreover, ICAM-1 is one of the initial markers during angiogenesis. The expression of ICAM-1 in vascular endothelial cells can promote the adhesion of cancer cells to the vascular endothelium (27). Then, cancer cells can secrete cytokines to promote the expression of VCAM-1 in endothelial cells, which can increase the production of macrophages and induce the secretion of VEGF and other factors, eventually promoting angiogenesis and assisting in the adhesion and metastasis of cancer cells (28). In this research, we found that MT-12 can inhibit the expression of ICAM-1 induced by PMA. Additionally, in vivo, MT-12 can reduce the expression of ICAM-1 in transplanted tumors. To summarize the above information, the anti-adhesion effect of MT-12 may be mediated by suppressing ICAM-1 expression.
Finally, in nude mouse models of subcutaneous tumors, after almost 1 month of MT-12 injection, the nude mice did not show severe adverse reactions, and the tumor shrank significantly. This phenomenon suggests that using MT-12 as an antitumor drug for bladder cancer might be viable.
Conclusions
In conclusion, this study revealed that MT-12 could inhibit the proliferation, invasion, and metastasis of BC in vitro and in vivo. The mechanism by which MT-12 affects BC cell growth and metastasis might relate to tumor angiogenesis by regulating MMP-9, VEGF, and ICAM-1. We believe the phenomenon is essential for the anti-tumor ability of MT-12. However, there are some deficiencies of this research. Ultimately, we concluded that the inhibition of cell angiogenesis might be an important mechanism for MT-12 to inhibit bladder cancer cells, but the inhibition of angiogenesis in a bladder tumor cell of MT-12 and its specific molecular mechanism still needs to be further explored.
Acknowledgments
Funding: Research supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.01.12). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The experiments on animals of this research were completed in Kunming Medical University’s Institutional Animal Care and Use Committee. The whole process strictly abides by the animal experiment guide.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Lin SR, Chang LS, Chang KL. Separation and structure-function studies of Taiwan cobra cardiotoxins. J Protein Chem 2002;21:81-6. [Crossref] [PubMed]
- Lee ML, Fung SY, Chung I, et al. King cobra (Ophiophagus hannah) venom L-amino acid oxidase induces apoptosis in PC-3 cells and suppresses PC-3 solid tumor growth in a tumor xenograft mouse model. Int J Med Sci 2014;11:593-601. [Crossref] [PubMed]
- Sun P, Ren XD, Zhang HW, et al. Serum from rabbit orally administered cobra venom inhibits growth of implanted hepatocellular carcinoma cells in mice. World J Gastroenterol 2003;9:2441-4. [Crossref] [PubMed]
- Becker AM, Keck RW, Murtagh DS Jr, et al. Prostatic involution after intraprostatic injection of cobra toxin. J Urol 2010;184:2192-6. [Crossref] [PubMed]
- Pointon A, Abi-Gerges N, Cross MJ, et al. Phenotypic profiling of structural cardiotoxins in vitro reveals dependency on multiple mechanisms of toxicity. Toxicol Sci 2013;132:317-26. [Crossref] [PubMed]
- Yang D, Wang J, Li J, et al. Effect of membrane toxin 12 isolated from Naja naja atra on proliferation and invasion of human bladder cancer EJ cells. Mol Med Rep 2012;5:266-9. [PubMed]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000;100:57-70. [Crossref] [PubMed]
- Klein T, Bischoff R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids 2011;41:271-90. [Crossref] [PubMed]
- Wieczorek E, Jablonska E, Wasowicz W, et al. Matrix metalloproteinases and genetic mouse models in cancer research: a mini-review. Tumour Biol 2015;36:163-75. [Crossref] [PubMed]
- Hegde PS, Wallin JJ, Mancao C. Predictive markers of anti-VEGF and emerging role of angiogenesis inhibitors as immunotherapeutics. Semin Cancer Biol 2018;52:117-24. [Crossref] [PubMed]
- Petrovic N. Targeting Angiogenesis in Cancer Treatments: Where do we Stand? J Pharm Pharm Sci 2016;19:226-38. [Crossref] [PubMed]
- Duro-Castano A, Gallon E, Decker C, et al. Modulating angiogenesis with integrin-targeted nanomedicines. Adv Drug Deliv Rev 2017;119:101-19. [Crossref] [PubMed]
- Sharma R, Sharma R, Khaket TP, et al. Breast cancer metastasis: Putative therapeutic role of vascular cell adhesion molecule-1. Cell Oncol (Dordr) 2017;40:199-208. [Crossref] [PubMed]
- Alexiou D, Karayiannakis AJ, Syrigos KN, et al. Serum levels of E-selectin, ICAM-1 and VCAM-1 in colorectal cancer patients: correlations with clinicopathological features, patient survival and tumour surgery. Eur J Cancer 2001;37:2392-7. [Crossref] [PubMed]
- Wang CH, Monette R, Lee SC, et al. Cobra cardiotoxin-induced cell death in fetal rat cardiomyocytes and cortical neurons: different pathway but similar cell surface target. Toxicon 2005;46:430-40. [Crossref] [PubMed]
- Guicciardi ME, Leist M, Gores GJ. Lysosomes in cell death. Oncogene 2004;23:2881-90. [Crossref] [PubMed]
- Wu M, Ming W, Tang Y, et al. The anticancer effect of cytotoxin 1 from Naja atra Cantor venom is mediated by a lysosomal cell death pathway involving lysosomal membrane permeabilization and cathepsin B release. Am J Chin Med 2013;41:643-63. [Crossref] [PubMed]
- Wang H, Yang D, Wang K, et al. Expression and potential role of chemokine receptor CXCR4 in human bladder carcinoma cell lines with different metastatic ability. Mol Med Rep 2011;4:525-8. [PubMed]
- Folkman J. The role of angiogenesis in tumor growth. Semin Cancer Biol 1992;3:65-71. [PubMed]
- Lucio-Eterovic AK, Piao Y, de Groot JF. Mediators of glioblastoma resistance and invasion during antivascular endothelial growth factor therapy. Clin Cancer Res 2009;15:4589-99. [Crossref] [PubMed]
- Ezhilarasan R, Jadhav U, Mohanam I, et al. The hemopexin domain of MMP-9 inhibits angiogenesis and retards the growth of intracranial glioblastoma xenograft in nude mice. Int J Cancer 2009;124:306-15. [Crossref] [PubMed]
- London CA, Hannah AL, Zadovoskaya R, et al. Phase I dose-escalating study of SU11654, a small molecule receptor tyrosine kinase inhibitor, in dogs with spontaneous malignancies. Clin Cancer Res 2003;9:2755-68. [PubMed]
- Shibuya M, Claesson-Welsh L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res 2006;312:549-60. [Crossref] [PubMed]
- Lichtenberger BM, Tan PK, Niederleithner H, et al. Autocrine VEGF signaling synergizes with EGFR in tumor cells to promote epithelial cancer development. Cell 2010;140:268-79. [Crossref] [PubMed]
- Wieczorek E, Jablonowski Z, Tomasik B, et al. MMP, VEGF and TIMP as prognostic factors in recurring bladder cancer. Clin Biochem 2015;48:1235-40. [Crossref] [PubMed]
- Benedicto A, Romayor I, Arteta B. Role of liver ICAM-1 in metastasis. Oncol Lett 2017;14:3883-92. [Crossref] [PubMed]
- Raimondi L, Amodio N, Di Martino MT, et al. Targeting of multiple myeloma-related angiogenesis by miR-199a-5p mimics: in vitro and in vivo anti-tumor activity. Oncotarget 2014;5:3039-54. [Crossref] [PubMed]


