
Effect of hepatitis B virus infection status on liver metastasis of nasopharyngeal carcinoma: a cohort study
Introduction
Nasopharyngeal carcinoma (NPC) is one of the most common malignancies in southern China, especially in the Guangdong province. In southern China, the incidence of NPC varies from 20 to 50 per 100,000 males (1). Because of the high radiosensitivity of NPC, radiotherapy is the standard treatment for early stage NPC. Comprehensive treatment based on concurrent chemoradiotherapy has become the standard therapeutic practice for intermediate- and late-stage NPC (2). However, NPC with distant metastasis still has a high mortality rate.
The liver is one of the most common sites for metastases in patients with NPC. Prognosis is poor for patients with NPC who have liver metastases due to the multifocality of liver metastasis (3). Recent research suggests that the response of the liver microenvironment to inflammation affects the colonization of circulating tumor cells and the occurrence of liver metastasis (4). This finding indicates that diseases such as hepatitis B or fatty liver disease, which cause inflammation in the liver, may influence the metastasis of malignancies to the liver.
Both NPC and hepatitis B are endemic to southern China. According to a survey, patients in China with hepatitis B virus (HBV) infection accounted for 10–12% of the total population, and chronic HBV infection accounted for a large portion of these infections (5). In patients with specific cancers, liver metastasis has been correlated with HBV infection, which affects patient prognosis. For example, in pancreatic cancer, patients who are HBsAg positive have a higher risk of liver metastasis than patients who are HBsAg negative (46.0% vs. 32.0%, P<0.05) (6). In contrast, a study of colorectal cancers reported that the occurrence of liver metastases was lower in patients with HBV infection than in those without HBV infection (13.5% vs. 27.1%, P<0.05) (7). A number of studies have reported the association between HBV infection and NPC. A retrospective, case-controlled study suggested that HBV infection increased the incidence of NPC (8). Liu et al. established that HBV infection had prognostic value in patients with locoregionally advanced NPC (9). However, the relationship between HBV infection status and liver metastasis of NPC was not discussed in those studies.
To date, few studies have explored the association of HBV infection status with liver metastasis of NPC. We conducted a multicenter, large-sample, retrospective study to clarify the influence of HBV infection status on liver metastasis in patients with NPC, the results of which may be helpful for improving the prognosis of patients with NPC.
Methods
Study populations
This was a two-center, retrospective cohort study of patients with NPC diagnosed by pathological examination at Nanfang Hospital or Zhujiang Hospital between 2005 and 2015. The exclusion criteria included the following: patients with distant metastases when first diagnosed, patients without regular tests for HBV, patients without regular liver imaging examinations, patients whose follow-up period was fewer than 30 days or 2 clinical encounters, patients with other malignancies, and patients with hepatitis C. A total of 1,367 patients with NPC were recruited for this study.
Clinical staging
All patients recruited for this study had undergone physical examinations, magnetic resonance imaging (MRI) of the nasopharynx, chest radiography, abdominal ultrasound (US), whole-body bone scans, and hematology tests. Patient information, including sex, age, time of diagnosis, treatment record and clinical stage, was obtained from the hospital clinical record systems. Tumor clinical stage was determined using the tumor node metastasis (TNM) classification system of the American Joint Committee on Cancer (7th edition, 2010).
HBV infection status
HBV infection was diagnosed using serologic tests for HBV. Serologic markers included HBsAg, hepatitis B surface antibody (HBsAb), hepatitis B e antigen (HBeAg) and hepatitis B e antibody (HBeAb), and hepatitis B core antibody (HBcAb). Patients were divided into four groups according to their HBV serologic markers: negative infection, chronic HBV infection, inactive HBV carrier and resolved HBV infection. Negative infection indicated that both HBsAg and HBcAb were negative. Chronic HBV infection was defined as being HBsAg and HBcAb positive and either HBeAg positive and/or HBV-DNA positive. Inactive HBV carriers were HBsAg positive and both HBeAg and HBV-DNA negative. Resolved HBV infection was defined as being mean HBsAg negative and either HBeAb or HBcAb positive. The specific HBV serologic marker status of the four different HBV infection groups are shown in Table 1.
Table 1
| HBV infection status | Negative infection | Chronic HBV infection | Inactive HBV carriers | Resolved HBV infection |
|---|---|---|---|---|
| HBV serologic marker status | HBsAg (−) and HBcAb (−) | HBsAg (+) and HBcAb (+) and either HBeAg (+) and/or HBV DNA (+) | HBsAg (+) and both HBeAg (−) and HBV DNA (−) | HBsAg (−) and either HBeAb (+) or HBcAb (+) |
−, negative; +, positive.
Patients’ treatment and follow-up
All patients were treated with radical radiotherapy. On the basis of the clinicians’ decisions, 77 of 1,367 patients (5.6%) were treated with radiotherapy only. In addition, 1,290 patients (94.4%) were treated with neoadjuvant, concurrent, or adjuvant chemotherapy. The regimens and dosages of chemotherapy were determined on the basis of the updated NCCN Guidelines for NPC. Serum liver function tests, including alanine transaminase (ALT), aspartate aminotransferase (AST), direct bilirubin (DB) and indirect bilirubin (IB), were conducted before and after chemotherapy.
Patients had follow-up appointments every 3 months during the first 2 years and then every 6 months thereafter. Patients received computed tomography (CT) or MRI examination of the chest and abdomen at regular intervals, and metastatic status was diagnosed by skilled radiologists. Liver metastases of NPC were diagnosed during each follow-up visit according to the examination of abdominal US liver imaging. However, CT or MRI could be used for further diagnosis of liver metastases when the results of an US were suspect. The follow-up time was calculated from the date when NPC was diagnosed to the date of liver metastasis or to the last liver imaging examination.
Statistical analysis
Statistical analyses were performed using SPSS (version 24.0; SPSS Inc., Chicago, Ill). Clinical characteristics of patients between the four groups, including sex, age, clinical stage, treatment, pathological type, ALT, AST, DB and IB, were compared using the chi-square test or Fisher’s exact test. The Kaplan-Meier method was utilized to calculate metastasis-free survival (MFS) in the survival analyses, and the differences between the survival curves of different groups were estimated with the log-rank test. Univariable Cox regression analyses were used to determine whether HBV infection status was related to liver metastasis of NPC. We used multivariate Cox regression analyses to evaluate the impact of HBV infection status on liver metastasis of NPC. All statistical analyses of clinical characteristics were two-sided, and P<0.05 was determined to be statistically significant.
Results
Baseline characteristics
In the current study, the average follow-up duration was 27.8 months (range, 1–145 months). Among the 1,367 patients, 1,039 (76.0%) were male. In total, 492 patients (36.0%) were negative for HBV infection. There were 123 patients (9.0%) with chronic HBV infection in the current study. Resolved HBV infection was found in 577 patients (42.2%), and 175 patients (12.8%) were inactive HBV carriers. Ultrasound scans confirmed that 216 patients (15.8%) had fatty liver disease.
The baseline characteristics of the four groups are displayed in Table 2. There was no significant difference among the four HBV infection status groups in age (≥60 years), T category, N category, TNM staging, pathological type, and IB. We observed no significant correlation between HBV infection status and fatty liver disease (P=0.097). The chronic HBV infection group had the highest proportion of male versus female patients (83.7%, P=0.012). The ALT levels, AST levels and DB levels were higher in the chronic HBV infection group than in the other groups (42.3%, P<0.01; 34.1%, P<0.01; 14.6%, P<0.01).
Table 2
| Characteristic | HBV status | P | |||
|---|---|---|---|---|---|
| Negative infection (n=492) | Chronic infection (n=123) | Inactive carriers (n=175) | Resolved infection (n=577) | ||
| Sex (%) | 0.012 | ||||
| Male | 353 (71.7) | 103 (83.7) | 141 (80.6) | 442 (76.6) | |
| Female | 139 (28.3) | 20 (16.3) | 34 (19.4) | 135 (23.4) | |
| Age (%) | 0.106 | ||||
| <60 | 436 (88.6) | 111 (90.2) | 158 (90.3) | 490 (84.9) | |
| ≥60 | 56 (11.4) | 12 (9.8) | 17 (9.7) | 87 (15.1) | |
| T category (%) | 0.835 | ||||
| T1 | 80 (16.3) | 22 (17.9) | 23 (13.1) | 86 (14.9) | |
| T2 | 134 (27.2) | 29 (23.6) | 53 (30.3) | 160 (27.7) | |
| T3 | 165 (33.5) | 45 (36.6) | 59 (33.7) | 215 (37.3) | |
| T4 | 113 (23.0) | 27 (21.9) | 40 (22.9) | 116 (20.1) | |
| N category (%) | 0.390 | ||||
| N0 | 57 (11.6) | 16 (13.0) | 21 (12.0) | 50 (8.7) | |
| N1 | 110 (22.4) | 26 (21.1) | 40 (22.9) | 123 (21.3) | |
| N2 | 263 (53.4) | 69 (56.1) | 102 (58.3) | 342 (59.3) | |
| N3 | 62 (12.6) | 12 (9.8) | 12 (6.8) | 62 (10.7) | |
| TNM staging (%) | 0.763 | ||||
| I | 18 (3.7) | 6 (4.9) | 3 (1.7) | 17 (3.0) | |
| II | 68 (13.8) | 16 (13.0) | 22 (12.6) | 74 (12.8) | |
| III | 247 (50.2) | 63 (51.2) | 100 (57.1) | 317 (54.9) | |
| IV | 159 (32.3) | 38 (30.9) | 50 (28.6) | 169 (29.3) | |
| Pathological types (%) | 0.277 | ||||
| Keratinizing squamous cell carcinoma | 19 (3.9) | 3 (2.4) | 10 (5.7) | 33 (5.7) | |
| Nonkeratinizing carcinoma | 473 (96.1) | 120 (97.6) | 165 (94.3) | 544 (94.3) | |
| Fatty liver (%) | 0.097 | ||||
| Yes | 73 (14.8) | 13 (10.6) | 24 (13.7) | 106 (18.4) | |
| No | 419 (85.2) | 110 (89.4) | 151 (86.3) | 471 (81.6) | |
| Chemotherapy (%) | 0.038 | ||||
| Radiotherapy only | 25 (5.1) | 14 (11.4) | 9 (5.1) | 29 (5.0) | |
| Chemoradiotherapy | 467 (94.9) | 109 (88.6) | 166 (94.9) | 548 (95.0) | |
| ALT (%) | <0.01 | ||||
| Elevated | 33 (6.7) | 52 (42.3) | 22 (12.6) | 37 (6.4) | |
| Normal | 459 (93.3) | 71 (57.7) | 153 (87.4) | 540 (93.6) | |
| AST (%) | <0.01 | ||||
| Elevated | 27 (5.5) | 42 (34.1) | 9 (5.1) | 12 (2.1) | |
| Normal | 465 (94.5) | 81 (65.9) | 166 (94.9) | 565 (97.9) | |
| DB (%) | <0.01 | ||||
| Elevated | 19 (3.9) | 18 (14.6) | 10 (5.7) | 27 (4.7) | |
| Normal | 473 (96.1) | 105 (85.4) | 165 (94.3) | 550 (95.3) | |
| IB (%) | 0.091 | ||||
| Elevated | 30 (6.1) | 11 (8.9) | 9 (5.1) | 22 (3.8) | |
| Normal | 462 (93.9) | 112 (91.1) | 166 (94.9) | 555 (96.2) | |
NPC, nasopharyngeal carcinoma; ALT, alanine aminotransferase; AST, aspartate aminotransferase; DB, direct bilirubin; IB, indirect bilirubin.
HBV infection status and liver metastasis
In this study, 105 patients with NPC had liver metastases during the follow-up period (7.7%). Among these, 51 patients were in the negative infection group, 13 patients were in the chronic HBV infection group, 7 patients were in the inactive HBV carrier group and 34 patients were in the resolved HBV infection group. The liver MFS curves of chronic HBV infection vs. negative infection, inactive carrier vs. negative infection and resolved HBV infection vs. negative infection are shown in Figure 1A,B,C. The 5-year liver MFS (94.1% vs. 80.8%, P=0.014) was higher in the inactive HBV carrier group than in the negative infection group. The 5-year liver MFS rates were 80.8% and 86.7% in patients with negative infection and resolved HBV infection, respectively (P=0.030). Compared with the negative infection group, the inactive HBV carrier status was related to liver metastasis in patients with NPC by univariate analysis (HR: 0.392; 95% CI: 0.178–0.863; P=0.020) and by multivariate analysis (HR: 0.404; 95% CI: 0.182–0.899; P=0.026). The resolved HBV infection group had a lower risk of liver metastasis of NPC than the negative infection group in the univariate analysis (HR: 0.621; 95% CI: 0.402–0.959; P=0.032) and the multivariate analysis (HR: 0.625; 95% CI: 0.400–0.975; P=0.039). However, there was no significant difference in the risk of liver metastases between the negative infection group and the chronic HBV infection group (P=0.880). Sex (male) (HR: 1.731; 95% CI: 1.030–2.909; P=0.038) and AST level (HR: 2.401; 95% CI: 1.408–4.092; P=0.001) were risk factors for liver metastasis. Fatty liver disease was not correlated with liver metastasis of NPC (P=0.615). The results of the univariate and multivariate analyses for liver metastasis are shown in Table 3.
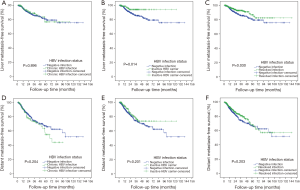
Table 3
| Parameter | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| HBV infection status | |||||
| Negative infection | Ref | – | Ref | – | |
| Chronic HBV infection | 0.954 (0.519–1.755) | 0.880 | 0.774 (0.379–1.585) | 0.484 | |
| Inactive carrier | 0.392 (0.178–0.863) | 0.020 | 0.404 (0.182–0.899) | 0.026 | |
| Resolved infection | 0.621 (0.402–0.959) | 0.032 | 0.625 (0.400–0.975) | 0.039 | |
| Sex | |||||
| Female | Ref | – | Ref | – | |
| Male | 1.731 (1.030–2.909) | 0.038 | 1.845 (1.091–3.118) | 0.022 | |
| Age | |||||
| <60 | Ref | – | Ref | – | |
| ≥60 | 1.331 (0.743–2.384) | 0.336 | 1.357 (0.746–2.467) | 0.317 | |
| T category | |||||
| T1 | Ref | – | Ref | – | |
| T2 | 2.010 (0.956–4.226) | 0.065 | 1.704 (0.800–3.628) | 0.167 | |
| T3 | 2.188 (1.056–4.537) | 0.035 | 1.894 (0.907–3.957) | 0.089 | |
| T4 | 2.974 (1.393–6.347) | 0.005 | 2.821 (1.306–6.092) | 0.008 | |
| N category | |||||
| N0 | Ref | – | Ref | – | |
| N1 | 1.751 (0.751–4.081) | 0.195 | 1.485 (0.617–3.573) | 0.377 | |
| N2 | 1.856 (0.839–4.102) | 0.127 | 1.590 (0.699–3.616) | 0.268 | |
| N3 | 4.894 (2.102–11.394) | <0.01 | 4.402 (1.825–10.618) | 0.001 | |
| Pathological types | |||||
| Keratinizing squamous cell carcinoma | Ref | – | Ref | – | |
| Nonkeratinizing carcinoma | 0.950 (0.417–2.166) | 0.902 | 0.637 (0.275–1.475) | 0.293 | |
| Chemotherapy | |||||
| Radiotherapy only | Ref | – | Ref | – | |
| Chemoradiotherapy | 2.149 (0.789–5.855) | 0.135 | 1.707 (0.588–4.951) | 0.325 | |
| Fatty liver | |||||
| No | Ref | – | Ref | – | |
| Yes | 1.143 (0.679–1.922) | 0.615 | 1.396 (0.809–2.409) | 0.231 | |
| ALT | |||||
| Normal | Ref | – | Ref | – | |
| Elevated | 1.392 (0.806–2.406) | 0.235 | 0.702 (0.328–1.505) | 0.363 | |
| AST | |||||
| Normal | Ref | – | Ref | – | |
| Elevated | 2.401 (1.408–4.092) | 0.001 | 2.921 (1.405–6.072) | 0.004 | |
| DB | |||||
| Normal | Ref | – | Ref | – | |
| Elevated | 1.316 (0.640–2.707) | 0.456 | 0.962 (0.390–2.373) | 0.934 | |
| IB | |||||
| Normal | Ref | – | Ref | – | |
| Elevated | 1.393 (0.703–2.761) | 0.342 | 1.218 (0.525–2.826) | 0.646 | |
NPC, nasopharyngeal carcinoma; ALT, alanine aminotransferase; AST, aspartate aminotransferase; DB, direct bilirubin; IB, indirect bilirubin.
HBV infection status and distant metastasis
Next, we decided to investigate the influence of HBV infection status on the distant metastasis of NPC. The metastatic sites of NPC included bone, liver, lung and other sites. Metastases were found in 241 patients during the follow-up period (17.6%). There were 97 patients with metastases in the negative infection group, 32 patients with metastases in the chronic HBV infection group, 26 patients with metastases in the inactive HBV carrier group and 86 patients with metastases in the resolved HBV infection group. The MFS curves of chronic HBV infection vs. negative infection, inactive carrier vs. negative infection and resolved HBV infection vs. negative infection are shown in Figure 1D,E,F. There was no significant difference in the 5-year DMFS (distant metastasis-free survival) rate between negative infection and other HBV infection statuses. We conducted univariate and multivariate analyses to evaluate the risk factors for distant metastasis of NPC. No significant difference was found in the risk of distant metastases between the negative infection group and the chronic HBV infection group (P=0.230). Similarly, inactive HBV carrier status was not associated with distant metastases compared with the negative infection group (P=0.228). Resolved HBV infection was not related to distant metastases of NPC compared with the negative infection group (P=0.206). Sex (male) (HR: 1.647; 95% CI: 1.177–2.306; P=0.004) and AST level (HR: 1.882; 95% CI: 1.276–2.776; P=0.001) were statistically significant predictors for distant metastasis of NPC. We did not find that fatty liver disease was significantly related to distant metastasis of NPC (P=0.599). The univariate and multivariate analyses for distant metastasis of NPC are shown in Table 4.
Table 4
| Parameter | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| HBV infection status | |||||
| Negative infection | Ref | – | Ref | – | |
| Chronic HBV infection | 1.277 (0.856–1.905) | 0.230 | 1.274 (0.799–2.032) | 0.309 | |
| Inactive carrier | 0.766 (0.497–1.182) | 0.228 | 0.794 (0.511–1.235) | 0.306 | |
| Resolved infection | 0.829 (0.620–1.109) | 0.206 | 0.852 (0.634–1.145) | 0.288 | |
| Sex | |||||
| Female | Ref | – | Ref | – | |
| Male | 1.647 (1.177–2.306) | 0.004 | 1.773 (1.261–2.492) | 0.001 | |
| Age | |||||
| <60 | Ref | – | Ref | – | |
| ≥60 | 1.242 (0.842–1.833) | 0.274 | 1.190 (0.799–1.772) | 0.393 | |
| T category | |||||
| T1 | Ref | – | Ref | – | |
| T2 | 1.726 (1.084–2.748) | 0.021 | 1.612 (1.001–2.594) | 0.049 | |
| T3 | 1.668 (1.052–2.645) | 0.030 | 1.517 (0.948–2.429) | 0.082 | |
| T4 | 3.211 (2.020–5.107) | <0.01 | 3.133 (1.949–5.037) | <0.01 | |
| N category | |||||
| N0 | Ref | – | Ref | – | |
| N1 | 1.428 (0.855–2.387) | 0.174 | 1.401 (0.821–2.390) | 0.216 | |
| N2 | 1.573 (0.978–2.530) | 0.062 | 1.479 (0.899–2.434) | 0.123 | |
| N3 | 3.297 (1.942–5.596) | <0.01 | 3.268 (1.871–5.709) | <0.01 | |
| Pathological types | |||||
| Keratinizing squamous cell carcinoma | Ref | – | Ref | – | |
| Nonkeratinizing carcinoma | 1.108 (0.620–1.980) | 0.729 | 0.890 (0.493–1.609) | 0.700 | |
| Chemotherapy | |||||
| Radiotherapy only | Ref | – | Ref | – | |
| Chemoradiotherapy | 1.569 (0.895–2.752) | 0.116 | 1.323 (0.721–2.428) | 0.367 | |
| Fatty liver | |||||
| No | Ref | – | Ref | – | |
| Yes | 0.905 (0.623–1.313) | 0.599 | 1.072 (0.729–1.578) | 0.723 | |
| ALT | |||||
| Normal | Ref | – | Ref | – | |
| Elevated | 1.113 (0.750–1.650) | 0.596 | 0.593 (0.343–1.022) | 0.060 | |
| AST | |||||
| Normal | Ref | – | Ref | – | |
| Elevated | 1.882 (1.276–2.776) | 0.001 | 2.499 (1.486–4.202) | 0.001 | |
| DB | |||||
| Normal | Ref | – | Ref | – | |
| Elevated | 0.842 (0.471–1.504) | 0.561 | 0.697 (0.350–1.385) | 0.302 | |
| IB | |||||
| Normal | Ref | – | Ref | – | |
| Elevated | 0.923 (0.538–1.584) | 0.771 | 0.984 (0.525–1.845) | 0.960 | |
NPC, nasopharyngeal carcinoma; ALT, alanine aminotransferase; AST, aspartate aminotransferase; DB, direct bilirubin; IB, indirect bilirubin.
Discussion
Recent studies have shown that inflammation caused by chronic liver diseases such as hepatitis B or fatty liver disease affects the development of liver metastasis. In a previous cohort study, we verified that fatty liver disease was a protective factor for liver metastasis of breast cancer (10). This result aroused our interest, and considering the large number of patients with NPC in China, we started this study to clarify the relationship between HBV infection status and liver metastasis of NPC. Our study results showed that sex (male) was an influencing factor for both distant metastasis and liver metastasis of NPC. Fatty liver disease was not related to either distant metastasis of NPC or liver metastasis of NPC in our study. HBV infection status was not associated with distant metastasis of NPC, but it was related to liver metastasis of NPC. Compared with patients in the negative infection group, the resolved HBV infection group and the inactive HBV carrier group had a lower risk of liver metastasis.
The evidence on whether HBV infection is related to liver metastasis of NPC is inconsistent. Lv et al. found that the presence of liver metastasis at diagnosis was significantly associated with HBV reactivation in NPC patients (OR, 7.19; P<0.01) (11). However, whether HBV infection was a risk factor that promoted liver metastasis of NPC was not explored in Lv’s research. Xu et al. reported that HBV infection increased the risk for distant recurrence of NPC, especially in the liver (12). However, Xu used only the HBsAg status to define hepatitis B infection, which had limitations in distinguishing different types of HBV infection status. It only identified patients with an active infection and inactive HBV carriers, but it did not identify those with a resolved HBV infection.
It has been reported that HBV infection status is closely associated with liver metastases of malignancies. In a previous study, Chen et al. examined four types of HBV infection status and found that chronic HBV infection decreased the risk of liver metastases in patients with advanced pancreatic ductal adenocarcinoma (13). Qiu et al. described that the occurrence of liver metastasis of colorectal cancer was significantly lower in the chronic HBV infection group (HR: 0.29, 95% CI: 0.12–0.72, P<0.01), inactive carrier group (HR: 0.36, 95% CI: 0.22–0.59, P<0.01) and resolved HBV infection group (HR: 0.63, 95% CI: 0.46–0.85, P<0.01) than in the non-infection group (14). In our study, we analyzed 1,367 clinical cases of NPC to examine the correlation between HBV infection status and liver metastasis. Our results suggested that there was no significant effect of HBV infection status on the occurrence of distant metastasis in NPC patients. The liver metastasis rate of NPC was lower in the inactive carrier and resolved HBV infection groups than in the negative infection group. It is reasonable to assume that an HBV infection without virus replication may affect liver metastasis of NPC. However, no significant difference in the liver metastasis rate was found between the negative infection group and the chronic HBV infection group, which differed from the observations for pancreatic and colorectal cancer. The development of tumors is promoted by inherent properties and is affected by the microenvironment (15). We hypothesize that the diversity of the inherent properties of tumors and the alterations that result from the interactions between tumors cells and the microenvironment may lead to different outcomes for liver metastases.
Based on these findings, we hypothesize that inactive HBV infection or resolved HBV infection changes the liver microenvironment by activating an inflammatory reaction in the liver, while HBV causes little injury to the liver. In the inactive HBV infection group and the resolved HBV infection group, HBV triggered an enhanced immune defense in the liver (16). The immune reaction caused by HBV is allegedly beneficial in preventing liver metastases in patients with NPC. Recent research has suggested that HBV infection promotes the secretion of cytokines such as transforming growth factor beta (TGF-β), tumor necrosis factor alpha (TNF-α), IL-1, IL-12, and platelet derived growth factor (PDGF). These cytokines regulate the extracellular matrix and directly prevent the adhesion of cancer cells (17). HBV infection also activates Kupffer cells and enhances cancer antigen presentation (18). During HBV infection, cytotoxic T lymphocytes (CTLs) and Kupffer cells are recruited to participate in the immune response in the liver. Tumor-specific CTLs are accompanied by Kupffer cells to perform antitumor functions by inhibiting proliferation and causing significant apoptosis of cancer cells (19). These changes prevent the colonization of circulating NPC cells in the liver and reduce the occurrence of liver metastasis. It is noteworthy that chronic HBV infection did not decrease the risk of liver metastasis of NPC in our study. We believe that in the chronic infection group, HBV was in the rapid replication stage, leading to apoptosis of numerous hepatocytes (20). Liver dysfunction weakens the immune response and the antitumor defense in the liver; therefore, the anti-metastatic effect is not enhanced in the liver.
To the best of our knowledge, this is the first cohort study to analyze the relationship between the different types of HBV infection status and liver metastasis of NPC. Compared with case-controlled studies, cohort studies are more suitable for assessing causal relationships. Our study recruited 1,367 patients with NPC from two hospitals, making our results representative and persuasive. In addition, our study excluded patients with distant metastases at NPC diagnosis because after tumor cells acquire the ability to invade distally, their progression is very different from that of the original tumor cells (21). Therefore, patients with non-metastatic NPC can better model the impact of HBV infection status on liver metastases by eliminating the effects of dissociated metastatic tumor cells on the results.
We must note that our study has several limitations. First, this report describes a retrospective study. Because a number of cases were excluded due to the loss of important information, selection bias may have existed and may have affected the final results of the analyses. Second, liver metastases were diagnosed by US, CT, and MRI instead of by pathological examination, which may have led to false-positive diagnoses. Moreover, only serologic HBV markers were used to define HBV infection status. The use of HBV DNA, such as covalently closed circular DNA (cccDNA), to define infection status could provide a more complete assessment and should be considered for use in future studies. Last but not least, there were too few cases in each group, and more data should be collected to make the result more convincing.
Conclusions
In this study, we observed that inactive HBV infections and resolved HBV infections were highly associated with a decreased risk of liver metastases in NPC patients compared with patients without HBV infections. The potential relationship of HBV infection status and liver metastasis of NPC needs to be investigated in further research.
Acknowledgments
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.01.25). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Institutional Review Boards of Nanfang Hospital and Zhujiang Hospital approved the study protocol (No. 2017-ZLZX-011), and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wei KR, Zheng RS, Zhang SW, et al. Nasopharyngeal carcinoma incidence and mortality in China in 2010. Chin J Cancer 2014;33:381-7. [PubMed]
- Chan AT. Nasopharyngeal carcinoma. Ann Oncol 2010;21:vii308-12. [Crossref] [PubMed]
- Ong YK, Heng DM, Chung B, et al. Design of a prognostic index score for metastatic nasopharyngeal carcinoma. Eur J Cancer 2003;39:1535-41. [Crossref] [PubMed]
- Mikuriya Y, Tashiro H, Kuroda S, et al. Fatty liver creates a pro-metastatic microenvironment for hepatocellular carcinoma through activation of hepatic stellate cells. Int J Cancer 2015;136:E3-13. [Crossref] [PubMed]
- Custer B, Sullivan SD, Hazlet TK, et al. Global epidemiology of hepatitis B virus. J Clin Gastroenterol 2004;38:S158-68. [Crossref] [PubMed]
- Wei XL, Qiu MZ, Chen WW, et al. The status of HBV infection influences metastatic pattern and survival in Chinese patients with pancreatic cancer. J Transl Med 2013;11:249. [Crossref] [PubMed]
- Song E, Chen J, Ou Q, et al. Rare occurrence of metastatic colorectal cancers in livers with replicative hepatitis B infection. Am J Surg 2001;181:529-33. [Crossref] [PubMed]
- Ye YF, Xiang YQ, Fang F, et al. Hepatitis B virus infection and risk of nasopharyngeal carcinoma in southern China. Cancer Epidemiol Biomarkers Prev 2015;24:1766-73. [Crossref] [PubMed]
- Liu X, Li X, Jiang N, et al. Prognostic value of chronic hepatitis B virus infection in patients with nasopharyngeal carcinoma: analysis of 1301 patients from an endemic area in China. Cancer 2014;120:68-76. [Crossref] [PubMed]
- Wu W, Chen J, Ye W, et al. Fatty liver decreases the risk of liver metastasis in patients with breast cancer: a two-center cohort study. Breast Cancer Res Treat 2017;166:289-97. [Crossref] [PubMed]
- Lv JW, Chen YP, Huang XD, et al. Hepatitis B virus screening and reactivation and management of patients with nasopharyngeal carcinoma: A large-scale, big-data intelligence platform-based analysis from an endemic area. Cancer 2017;123:3540-9. [Crossref] [PubMed]
- Xu T, Huang Z, Deng Y, et al. Clinical implications of hepatitis B viral infection in Epstein-Barr virus-associated nasopharyngeal carcinoma. J Clin Virol 2015;64:64-71. [Crossref] [PubMed]
- Chen Q, Ning Z, Wang L, et al. Is chronic hepatitis B infection a protective factor for the progression of advanced pancreatic ductal adenocarcinoma? An analysis from a large multicenter cohort study. Oncotarget 2016;7:85603-12. [Crossref] [PubMed]
- Qiu HB, Zhang LY, Zeng ZL, et al. HBV infection decreases risk of hepatic metastasis in patients with colorectal cancer: a cohort study. World J Gastroenterol 2011;17:804-8. [Crossref] [PubMed]
- Mueller MM, Fusenig NE. Friends or foes - bipolar effects of the tumour stroma in cancer. Nat Rev Cancer 2004;4:839-49. [Crossref] [PubMed]
- Ferrari C. HBV and the immune response. Liver Int 2015;35:121-8. [Crossref] [PubMed]
- Okuno K, Hirai N, Lee YS, et al. Involvement of liver-associated immunity in hepatic metastasis formation. J Surg Res 1998;75:148-52. [Crossref] [PubMed]
- Schuurman B, Heuff G, Beelen RH, et al. Enhanced human Kupffer cell-mediated cytotoxicity after activation of the effector cells and modulation of the target cells by interferon-gamma: a mechanistic study at the cellular level. Cell Immunol 1995;165:141-7. [Crossref] [PubMed]
- Wang Z, Li P, Xu Q, et al. Potent Antitumor Activity Generated by a Novel Tumor Specific Cytotoxic T Cell. PLoS One 2013;8:e66659. [Crossref] [PubMed]
- Rawat S, Clippinger AJ, Bouchard MJ. Modulation of apoptotic signaling by the hepatitis B virus X protein. Viruses 2012;4:2945-72. [Crossref] [PubMed]
- Walsh JE, Lathers DM, Chi AC, et al. Mechanisms of tumor growth and metastasis in head and neck squamous cell carcinoma. Curr Treat Options Oncol 2007;8:227-38. [Crossref] [PubMed]


