
miR-30b suppresses the progression of breast cancer through inhibition of the PI3K/Akt signaling pathway by targeting Derlin-1
Introduction
Breast cancer is one of the most common cancers in female patients. The mortality rate of this disease in females is second only to that of lung cancer, causing an estimated 465,000 deaths worldwide each year (1-3). Metastasis is a common cause for these deaths. Therefore, it is imperative to find potential therapeutic targets to suppress tumor metastasis and to establish novel treatment programs for breast cancer.
MicroRNAs (miRNAs) are a class of small non-coding RNAs, which can modulate the expression of target genes by binding to the 3'UTR of messenger RNA (mRNA) to trigger mRNA degradation or inhibit translation. miRNAs are involved in cell proliferation, differentiation, apoptosis, and signal transduction by regulating gene posttranscriptional expression (4-7). In recent years, numerous reports have revealed that miRNAs play essential roles as oncogenes or tumor suppressors in the initiation, progression and metastasis of tumors, including breast cancer (8-11). As a result, miRNAs are considered potential therapeutic targets for breast cancer therapy. It has been confirmed that miR-30b is down-regulated in bladder cancer (12), gastric cancer (13) and breast cancer cells (14). Furthermore, miR-30b can modulate cell proliferation and induce cell cycle arrest in breast cancer cells (14). However, the specific role of miR-30b on breast cancer metastasis remains unknown; we aim to elucidate its involvement in metastasis.
In this study, we aimed to investigate the role of miR-30b in tumor progression and metastasis of breast cancer cell in vitro. We confirmed that miR-30b modulated cell proliferation, migration, invasion, and apoptosis through inhibition of the PI3K/Akt signaling pathway by targeting Derlin-1 in breast cancer cells. Our findings indicated that miR-30b may play a role as a novel tumor suppressor gene in breast cancer.
Methods
Cells culture and transfection
We obtained the human breast cancer cell lines SKBR3 and MDA-MB-231 from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). SKBR3 cells were cultured in McCoy’s 5a Medium Modified (Thermo Fisher Scientific, Waltham, MA, USA) containing 10% FBS (Gibco, Thermo Fisher Scientific), and MDA-MB-231 cells were cultured in Leibovitz’s L-15 Medium (Thermo Fisher Scientific, Waltham, MA, USA) containing 10% FBS (Gibco) at 37 °C with 5% CO2. Cells were transiently transfected with the pCMV-miR-30b plasmid (Ribobio, Guangzhou, China) or negative control (NC) plasmid (pCMV-miR) using Lipofectamine 2000 (Invitrogen, Thermo Fisher Scientific) following the manufacturer’s protocol. After 24 hours of incubation, miR-30b expression could be observed and the subsequent experiment was carried out. The siRNA-Derlin-1 sequence (Oligobio, Beijing, China) and the pcDNA3.1-Derlin-1 (Oligobio) were used to knock down or up-regulate the expression of Derlin-1. This study was approved by the Research Ethics Committee of Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine (No. 2018-142-01).
Real-time polymerase chain reaction (qRT-PCR)
Following 24 hours of transfection, total RNA was extracted using Ultrapure RNA Kit (CWBIO, Beijing, China) and reverse transcribed into cDNA using miRNA cDNA Synthesis Kit (CWBIO). The relative expression level of miR-30b was subsequently detected using the miRNA qPCR Assay Kit (CWBIO). The primers for mature miR-30b and U6 RNA were obtained from Ribobio. The comparative Ct (ΔΔCt) method was adopted to calculate the obtained qRT-PCR data.
Proliferation and viability assay
CCK8 assay was utilized to detect cell proliferation and viability of SKBR3 and MDA-MB-231 cells. After being transfected for 24 hours, cells were seeded into each well of a 96-well plate at a density of 1×103 cells per well. 10 µL of CCK8 reagent (Beijing Solarbio Science & Technology, Beijing, China) was added into each well to culture at 37 °C for 90 minutes. We then determined the optical density (OD) value of the excited light every 24 hours by using the enzyme-linked immunosorbent assay at 450 nm.
Colony formation assay was also performed to detect cell proliferation. Transfected cells at the logarithmic growth phase were seeded into 6 cm plates at a density of 500 cells per well to culture at 37 °C with 5% CO2 until cells had formed sufficiently large colonies. After that, cells were fixed with 4% paraformaldehyde for 30 minutes followed by staining with 500 µL GIEMSA (DingGuo Bio, Cat#AR-0752, Shanghai, China) for 10 minutes. The colonies were counted and photographed.
Western blot analysis
After being transfected for 48 hours, cells were lysed with ice-cold RIPA Lysis Buffer (CWBIO) and the protein concentration was detected using a BCA Protein Assay Kit (Beyotime, Shanghai, China). Thereafter, 20 µg of protein of each sample was separated by 10% SDS-PAGE gel, and then transferred to the polyvinylidene fluoride membrane (PVDF, Millipore, Billerica, MA, USA). The primary antibodies anti-CDK4 (Cat#11026-1-AP, Proteintech Group Inc., Rosemont, IL, USA), anti-CDK6 (Cat#14052-1-AP, Proteintech Group Inc.), anti-Active Caspase-3 p17-specific (Cat#25546-1-AP, Proteintech Group Inc.), anti-Notch1 (Cat#10062-2-AP, Proteintech Group Inc.), anti-Bcl-2 (Cat#ab32124, Abcam, Cambridge, United Kingdom), anti-Bax (Cat#ab32503, Abcam), anti-Caspase-9 (Cat#ab32539, Abcam), anti-p-Akt (Cat#ab81283, Abcam), anti-Akt (Cat#ab32505, Abcam), anti-mTOR (Cat#ab2732, Abcam), anti-p-mTOR (Cat#ab131538, Abcam), anti-P70S6K (Cat#ab32529, Abcam), anti-Cyclin D1 (Cat#ab40754, Abcam), anti-Derlin-1 (Cat#SAB4200148, Sigma) and anti-GAPDH (Cat#ab8245, Abcam) were added to the PVDF membrane in blocking solution and incubated at 4 °C overnight. Next, the secondary antibodies (Proteintech Group Inc.) were added to the PVDF membrane and incubated for 1 hour, and an enhanced chemiluminescence kit (CWBIO) was utilized for detection of signal development. Image J software was used to estimate the density of each band in order to analyze the relative expression of each protein.
Migration and invasion assays
For invasion assay: Cell invasion experiments were performed using Transwell chambers (Millipore) coated with Matrigel (BD Biosciences, NJ, USA). Following 24 hours of transfection, cells were resuspended in serum-free culture medium. About 1×104 cells were transferred into the top chamber, then the complete medium with 10% FBS was added into the lower chamber and cultured for 24 hours. The non-invading cells were removed with a cotton swab and invasive cells were fixed with 4% paraformaldehyde. Then, the cells were stained with 0.1% crystal violet for 5 minutes. The invaded cells were captured and counted under the microscope.
For migration assay: a method similar to the invasion assay was employed; the only differences were that Matrigel was absent and the number of cells was 5×103.
Apoptosis assay
Annexin V-FITC-PI assay was used to detect the rate of apoptosis of SKBR3 and MDA-MB-231 cells. Following 24 hours of transfection, cells were collected and resuspended in 1× binding buffer at a density of [1–5]×106 cells/mL. Then 100 µL of cell suspension was stained using an Annexin V-FITC-PI apoptosis detection kit (4A Biotech Co., Beijing, China) for incubation at room temperature in the dark. The samples were analyzed using a FACScalibur instrument. The rate of apoptosis was analyzed using BD FACSDiva software.
Dual-luciferase reporter assay
The precise target of miR-30b was identified using a dual-luciferase reporter assay. 5×105 cells were incubated into 24-well culture plates. After 24 hours of incubation, luciferase reporter plasmids, pmirGLO-Derlin-1-3'UTR [wild type (wt)] or pmirGLO-Derlin-1-3'UTR mutant (mut), were co-transfected with pCMV-miR-30b (miR-30b), pCMV-miR (NC) or miR-30b inhibitor (Ribobio) by Liposome 2000 into the SKBR3 and MDA-MB-231 cells. Following 48 hours of transfection, the luciferase activity was measured.
Statistical analysis
The data were represented from three independent experiments and expressed as means ± standard deviation (SD). SPSS 18.0 software was utilized to calculate the statistical analysis. Comparisons between two groups were analyzed using the student t-test, and one-way ANOVA was performed to compare three or more groups. Differences were considered statistically significant for values of P<0.05.
Results
Overexpression of miR-30b inhibits proliferation and viability of breast cancer cells
In order to investigate the effects of miR-30b on breast cancer progression, the pCMV-MIR- miR-30b vector was transfected into breast cancer cell lines SKBR3 and MDA-MB-231 to up-regulate the expression of miR-30b, and the pCMV-MIR vector was used as the NC. As shown in Figure 1A, in comparison with the NC group, the expression of miR-30b was significantly up-regulated both in SKBR3 and MDA-MB-231 cells. CCK8 assay showed that up-regulation of miR-30b significantly reduced cell viability in SKBR3 cells compared to the NC group (Figure 1B). Similar inhibition caused by up-regulation of miR-30b was also observed in MDA-MB-231 cells (Figure 1C). We further detected the effect of miR-30b on colony formation efficiency of breast cancer cells. The results showed that compared to the NC group, the colony formation ability of miR-30b up-regulated cells was decreased (Figure 1D). Western blot results also suggested that the expression of cell cycle-related proteins, CDK4 and CDK6, which play critical roles in cell proliferation, were both decreased in miR-30b overexpressed cells (Figure 1E). These results together indicated that up-regulated miR-30b inhibited cell proliferation and viability of breast cancer cells in vitro.
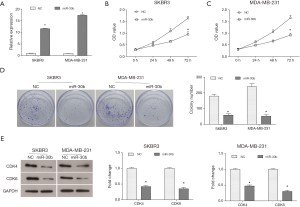
Overexpression of miR-30b reduces migration and invasion abilities of breast cancer cells
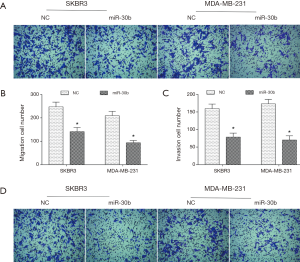
It is well known that one of the most important features of tumors is metastasis. Therefore, we examined the effects of miR-30b on breast cancer metastasis. As shown in Figure 2A,B, overexpression of miR-30b reduced the migration ability of SKBR3 and MDA-MB-231 cells. Furthermore, compared to the NC, the cell invasion ability was also significantly decreased in miR-30b overexpressed cells (Figure 2C,D). All in all, these data suggested an inhibitory role of miR-30b on the migration and invasion of breast cancer cells in vitro.
Overexpression of miR-30b promotes apoptosis of breast cancer cells
Another notable hallmark of tumors is uncontrolled apoptosis (15). Flow cytometry assay was performed to detect the effects of miR-30b on cell apoptosis of breast cancer. Annexin V and PI were used to stain SKBR3 and MDA-MB-231 cells, and the early and late apoptotic cells were identified by Annexin V-positive and PI-negative staining. We observed that up-regulation of miR-30b significantly promoted the rate of apoptosis in comparison with the NC group for both SKBR3 and MDA-MB-231 cells (Figure 3A,B). In order to determine the mechanism of apoptosis induction by overexpression of miR-30b in breast cancer cells, we detected the expression of apoptosis-related proteins. Western blot results showed a significant decrease in the expression of anti-apoptotic protein Bcl-2 and a notable increase in the expression of pro-apoptotic proteins Bax, Caspase-9 and active Caspase-3 in SKBR3 and MDA-MB-231 cells which were transfected with miR-30b (Figure 3C,D,E). These data suggested that overexpression of miR-30b induced apoptosis by regulating the Bcl-2/Bax axis and Caspase cascade in breast cancer cells.

miR-30b directly targets Derlin-1 in breast cancer cells
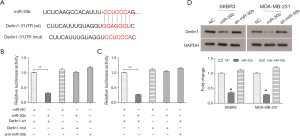
As previously mentioned, miRNA is able to bind the 3'UTR of mRNA to regulate expression of the target gene. Bioinformatics analysis from Targetcan revealed that the Derlin-1 3'UTR has a putative binding site for miR-30b (Figure 4A). To confirm this, pmirGLO-Derlin-1-3'UTR (wt) or pmirGLO-Derlin-1-3'UTR mutant (mut) plasmid were constructed for co-transfection with pCMV-MIR-miR-30b or miR-30b inhibitor (anti-miR-30b) into SKBR3 and MDA-MB-231 cells. The dual-luciferase reporter assay showed that the luciferase activity in miR-30b+ Derlin-1-wt group was significantly decreased compared to the miR-NC+ Derlin-1-wt group (P<0.01, Figure 4B), while the mutant counterpart of Derlin-1 blocked the decrease caused by miR-30b, and the anti-miR-30b did not reduce the expression of Derlin-1, suggesting that Derlin-1 was a target gene for miR-30b in SKBR3 cells. Similar results were also observed in MDA-MB-231 cells (Figure 4C). As shown in Figure 4D, miR-30b significantly inhibited the protein expression of Derlin-1, while transfection with the miR-30b inhibitor did not impact its expression, further suggesting that Derlin-1 is a target gene for miR-30b and that it could be regulated by miR-30b at the post-transcriptional level.
Overexpression of miR-30b inhibits the PI3K/Akt pathway by targeting Derlin-1 in breast cancer cells
The PI3K/Akt signaling pathway plays a pivotal role in multiple cellular processes through regulating its downstream proteins, including cell cycle-related proteins and Bcl-2-related proteins. It has been confirmed that the PI3K/Akt pathway is frequently activated in breast cancer (16). Our study found that transfection with miR-30b had no effect on the expression of total Akt and mTOR in SKBR3 and MDA-MB-231 cells (Figure 5A). Nevertheless, overexpression of miR-30b significantly reduced the level of the phosphorylated forms p-Akt and p-mTOR (Figure 5A). Meanwhile, the expression of the downstream proteins, Cyclin D1 and P70S6K, were both decreased in miR-30b overexpressed cells. These results indicated that miR-30b could suppress the activation of the PI3K/Akt signaling pathway in breast cancer in vitro.
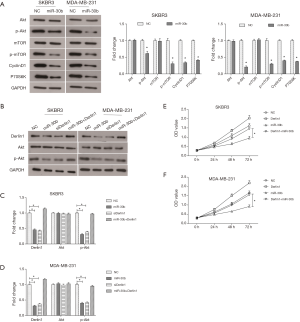
Further studies were employed to investigate the mechanism underlying the inhibitory effects of miR-30b on the progression of breast cancer. As the Western blot results suggested, silencing Derlin-1 significantly decreased the phosphorylation of Akt in SKBR3 and MDA-MB-231 cells, which was consistent with the inhibitory effect of miR-30b on p-Akt (Figure 5B,C,D). Moreover, up-regulation of Derlin-1 compensated for the miR-30b induced decrease of p-Akt in breast cancer cells (Figure 5B,C,D). Furthermore, when compared with the miR-30b overexpression group, the proliferation abilities of SKBR3 and MDA-MB-231 cells was significantly reversed in the miR-30b+ Derlin-1 group (P<0.05, Figure 5E,F). Overall, theses data indicated that miR-30b could inhibit the PI3K/Akt pathway through targeting of Derlin-1, resulting in inhibition of breast cancer cell growth.
Discussion
An increasing amount of evidence has confirmed that multiple miRNAs play roles as suppressors or oncogenes in the progression of tumor. For example, the miR-200 family is considered to be associated with the epithelial-mesenchymal transition (EMT) and plays an essential role in tumor progression by modulating EMT in breast cancer (17,18). The miR-17/20 cluster, correlated with loss of heterozygosity in breast cancer, is revealed to act as a suppressor in the progression of breast cancer through down-regulation of AIB1 and Cyclin D1 (19). It has been confirmed that miR-30b is down-regulated in multiple tumor tissues and cells (12-14,20,21), including breast cancer (14). For instance, Li et al. demonstrates that miR-30b could suppress cell growth and invasion through targeting homeobox A1 in esophageal cancer (20). Up-regulated miR-30b could inhibit tumor growth and promote cell apoptosis in gastric cancer (13). Our data also showed that overexpression of miR-30b significantly suppressed cell proliferation and viability of SKBR3 and MDA-MB-231 cells in vitro, which was consistent with previous studies (14,22). Although the inhibitory effect of miR-30b on the proliferation of breast cancer cells has already been identified, the role of miR-30b in the metastasis of breast cancer and the specific mechanism remains unknown.
Invasion and metastasis are the basic biological hallmarks of malignant tumors, leading to treatment failure and ultimately the death of patients (23,24). In this study, we found that overexpression of miR-30b can significantly inhibit the migration and invasion abilities of SKBR3 and MDA-MB-231 cells, suggesting that miR-30b acts as a tumor suppressor in the growth and invasion of breast cancer in vitro.
Apoptosis is a common regulatory mechanism used to control cell growth (25). Once the balance between cell survival and death is broken, it will result in cancer (26). The intrinsic mitochondrial pathway is one of the main mechanisms of apoptosis (27), however, cancer cells can evade this tightly regulated cell death process through regulation of anti-apoptotic or pro-apoptotic molecules (28). Therefore, promoting cancer cell apoptosis is an important method for cancer therapy. The Caspase and Bcl-2 protein families are key proteins related to apoptosis. Specifically, anti-apoptotic Bcl-2, pro-apoptotic Bax, Caspase-3, and Caspase-9 are critical factors involved in the initiation of the mitochondrial-dependent apoptosis pathway (29-31). Overexpression of Bax can trigger the release of mitochondrial proteins that activate Caspase-9. Activated Caspase-9 can catalyze the activation of Caspase-3 which eventually leads to apoptosis (32,33). Up-regulated expression of Bcl-2 has been reported in a great majority of primary breast cancer (34,35). Zhu et al. discovered that overexpression of miR-30b induces cell apoptosis of gastric cancer cells, suggesting that the suppression of tumor growth via miR-30b might occur via the induction of apoptosis (13). In the current report, overexpression of miR-30b enhanced cell apoptosis of SKBR3 and MDA-MB-231 cells, and decreased expression of Bcl-2 and increased expression of Bax, Caspase-9 and active Caspase-3 (Figure 3). Tormo et al. also discovered that miR-30b mimic can raise the expression of Caspase-3 in breast cancer BT474 cells (22). The findings described above indicate that miR-30b triggers cell apoptosis via the mitochondrial pathway through regulation of the Bcl-2/Bax axis and Caspase cascade in breast cancer in vitro.
It is well known that miRNAs can regulate the expression of target genes at the post-transcriptional level by binding to the 3'UTR of the target gene. Therefore, finding the target genes for miR-30b in breast cancer cells is very important for investigating the mechanism of miR-30b involvement in breast cancer progression. Bioinformatics analysis from Targetcan showed that Derlin-1 3'UTR has a putative binding site for miR-30b. Additionally, our data showed that miR-30b could down-regulate the expression of Derlin-1 in a post-transcriptional manner by employing the dual-luciferase reporter and western blot assays, indicating that Derlin-1 is a novel target for miR-30b. An increasing amount of evidence has revealed that Derlin-1 functions as an oncogene in multiple cancers. Dong et al. reported that Derlin-1 is up-regulated in bladder cancer and its overexpression promotes proliferation and invasion of bladder cancer cells (36). In glioma cells, Derlin-1 is also found to be involved in tumor progression by promoting cell proliferation, migration and invasion (37). A previous study found that Derlin-1 is over-expressed in breast cancer and associated with tumor grade and lymph node metastasis (38). Based on these studies, our data indicated that regulation of Derlin-1 might be involved in the function of miR-30b in breast cancer.
In order to further investigate the related mechanism involving miR-30b on the progression and metastasis of breast cancer in vitro, we examined an important relevant intracellular signaling pathway. It is well known that the PI3K/Akt pathway plays an essential role in numerous cellular activities, including proliferation, survival and metastasis (39). It has been demonstrated that the PI3K/Akt signaling pathway is mostly overactive in breast cancer and its activation is closely related to drug resistance in breast cancer therapy (40,41). In the present study, miR-30b suppressed the activation of the PI3K/Akt pathway by decreasing the level of the phosphorylated form of Akt and mTOR (Figure 5A). Consistent with this, the expression of P70S6K and Cyclin D1, critical downstream cell proliferation and cell cycle-related proteins, also decreased, suggesting that the PI3K/Akt signaling pathway might be involved in the function of miR-30b in breast cancer. Moreover, we observed that depletion of Derlin-1 could inhibit the PI3K/Akt pathway by decreasing the phosphorylation of Akt, meanwhile, up-regulation of Derlin-1 could restore the inhibition on Akt phosphorylation caused by miR-30b (Figure 5B). A recent study has revealed that Derlin-1 could up-regulate the phosphorylation of Akt and interact with PI3K p110α in bladder cancer cells (36). Subsequently, the author discovered that up-regulation of Derlin-1 could promote the activation of the PI3K/Akt pathway, meanwhile silencing of Derlin-1 results in inhibition of esophageal squamous cell carcinoma cells (42). Based on these findings, our data indicate that miR-30b inhibits the PI3K/Akt signaling pathway by targeting Derlin-1. In addition, CCK8 assay showed that Derlin-1 could partially reverse the inhibition of cell proliferation in SKBR3 and MDA-MB-231 cells caused by miR-30b. This data in its entirety indicates that miR-30b inhibits proliferation of breast cancer cells through down-regulating the PI3K/Akt signaling pathway by targeting Derlin-1.
Conclusions
In summary, this study demonstrates that miR-30b has the potential to inhibit the progression and metastasis of breast cancer in vitro. This is thought to occur through down-regulation of the PI3K/Akt signaling pathway by targeting Derlin-1. Therefore, miR-30b is a potential novel target for breast cancer therapy.
Acknowledgments
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.01.21). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Research Ethics Committee of Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine (No. 2018-142-01).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11. [Crossref] [PubMed]
- Donepudi MS, Kondapalli K, Amos SJ, et al. Breast cancer statistics and markers. J Cancer Res Ther 2014;10:506. [PubMed]
- DeSantis C, Ma J, Bryan L, et al. Breast cancer statistics, 2013. CA Cancer J Clin 2014;64:52. [Crossref] [PubMed]
- Bartel DP. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell 2004;116:281-97. [Crossref] [PubMed]
- Bartel DP. MicroRNAs: Target Recognition and Regulatory Functions. Cell 2009;136:215. [Crossref] [PubMed]
- Ambros V. The functions of animal microRNAs. Nature 2004;431:350. [Crossref] [PubMed]
- Ebert MS, Sharp PA. Roles for microRNAs in conferring robustness to biological processes. Cell 2012;149:515. [Crossref] [PubMed]
- Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer 2006;6:259-69. [Crossref] [PubMed]
- Pencheva N, Tavazoie SF. Control of Metastatic Progression by microRNA Regulatory Networks. Nat Cell Biol 2013;15:546-54. [Crossref] [PubMed]
- Shen Y, Ye YF, Ruan LW, et al. Inhibition of miR-660-5p expression suppresses tumor development and metastasis in human breast cancer. Genet Mol Res 2017;16: [Crossref] [PubMed]
- Li X, Li Y, Lu H. MiR-1193 suppresses proliferation and invasion of human breast cancer cells through directly targeting IGF2BP2. Oncol Res 2017;25:579-85. [Crossref] [PubMed]
- Wszolek MF, Rieger-Christ KM, Kenney PA, et al. A MicroRNA expression profile defining the invasive bladder tumor phenotype. Urol Oncol 2011;29:794. [Crossref] [PubMed]
- Zhu ED, Li N, Li BS, et al. miR-30b, down-regulated in gastric cancer, promotes apoptosis and suppresses tumor growth by targeting plasminogen activator inhibitor-1. PLoS One 2014;9:e106049. [Crossref] [PubMed]
- Ichikawa T, Sato F, Terasawa K, et al. Trastuzumab Produces Therapeutic Actions by Upregulating miR-26a and miR-30b in Breast Cancer Cells. PLoS One 2012;7:e31422. [Crossref] [PubMed]
- Cotter TG. Apoptosis and cancer: the genesis of a research field. Nat Rev Cancer 2009;9:501-7. [Crossref] [PubMed]
- López-Knowles E, O’Toole SA, Mcneil CM, et al. PI3K pathway activation in breast cancer is associated with the basal-like phenotype and cancer-specific mortality. Int J Cancer 2010;126:1121-31. [Crossref] [PubMed]
- Li X, Roslan S, Johnstone CN, et al. MiR-200 can repress breast cancer metastasis through ZEB1-independent but moesin-dependent pathways. Oncogene 2014;33:4077-88. [Crossref] [PubMed]
- Manavalan TT, Teng Y, Litchfield LM, et al. Reduced Expression of miR-200 Family Members Contributes to Antiestrogen Resistance in LY2 Human Breast Cancer Cells. PLoS One 2013;8:e62334. [Crossref] [PubMed]
- Yu Z, Xu Z, Disante G, et al. miR-17/20 sensitization of breast cancer cells to chemotherapy-induced apoptosis requires Akt1. Oncotarget 2014;5:1083-90. [Crossref] [PubMed]
- Li Q, Xuan Z, Ning L, et al. miR-30b inhibits cancer cell growth, migration, and invasion by targeting homeobox A1 in esophageal cancer. Biochem Biophys Res Commun 2017;485:506. [Crossref] [PubMed]
- Chen S, Li P, Yang R, et al. microRNA-30b inhibits cell invasion and migration through targeting collagen triple helix repeat containing 1 in non-small cell lung cancer. Cancer Cell Int 2015;15:85. [Crossref] [PubMed]
- Tormo E, Adamartigues A, Ballester S, et al. The role of miR-26a and miR-30b in HER2+ breast cancer trastuzumab resistance and regulation of the CCNE2 gene. Sci Rep 2017;7:41309. [Crossref] [PubMed]
- Zhao L, Zhao Y, He Y, et al. miR-19b promotes breast cancer metastasis through targeting MYLIP and its related cell adhesion molecules. Oncotarget 2017;8:64330-43. [PubMed]
- Dai X, Xiang L, Li T, et al. Cancer Hallmarks, Biomarkers and Breast Cancer Molecular Subtypes. J Cancer 2016;7:1281. [Crossref] [PubMed]
- Tomei LD, Cope FO. Apoptosis: the molecular basis of cell death. Cold Spring Harbor: Cold Spring Harbor Laboratory; 1991.
- Koff JL, Ramachandiran S, Bernal-Mizrachi L. A time to kill: targeting apoptosis in cancer. Int J Mol Sci 2015;16:2942. [Crossref] [PubMed]
- Parrish AB, Freel CD, Kornbluth S. Cellular mechanisms controlling caspase activation and function. Cold Spring Harb Perspect Biol 2013;5:239-49. [Crossref] [PubMed]
- Igney FH, Krammer PH. Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer 2002;2:277. [Crossref] [PubMed]
- Gui D, Guo Y, Feng W, et al. Astragaloside IV, a Novel Antioxidant, Prevents Glucose-Induced Podocyte Apoptosis In Vitro and In Vivo. PLoS One 2012;7:e39824. [Crossref] [PubMed]
- Martinou JC, Youle RJ. Mitochondria in Apoptosis: Bcl-2 family Members and Mitochondrial Dynamics. Dev Cell 2011;21:92. [Crossref] [PubMed]
- Wu R, Tang S, Wang M, et al. MicroRNA-497 Induces Apoptosis and Suppresses Proliferation via the Bcl-2/Bax-Caspase9-Caspase3 Pathway and Cyclin D2 Protein in HUVECs. PLoS One 2016;11:e0167052. [Crossref] [PubMed]
- Végran F, Boidot R, Solary E, et al. A short caspase-3 isoform inhibits chemotherapy-induced apoptosis by blocking apoptosome assembly. PLoS One 2011;6:e29058. [Crossref] [PubMed]
- Cain K, Bratton SB, Cohen GM. The Apaf-1 apoptosome: a large caspase-activating complex. Biochimie 2002;84:203. [Crossref] [PubMed]
- Dawson SJ, Makretsov N, Blows FM, et al. BCL2 in breast cancer: a favourable prognostic marker across molecular subtypes and independent of adjuvant therapy received. Br J Cancer 2010;103:668. [Crossref] [PubMed]
- Merino D, Lok SW, Visvader JE, et al. Targeting BCL-2 to enhance vulnerability to therapy in estrogen receptor-positive breast cancer. Oncogene 2016;35:1877. [Crossref] [PubMed]
- Dong Q, Lin F, Yue Z, et al. Derlin-1 overexpression confers poor prognosis in muscle invasive bladder cancer and contributes to chemoresistance and invasion through PI3K/AKT and ERK/MMP signaling. Oncotarget 2017;8:17059-69. [PubMed]
- Zheng J, Liu X, Xue Y, et al. TTBK2 circular RNA promotes glioma malignancy by regulating miR-217/HNF1β/Derlin-1 pathway. J Hematol Oncol 2017;10:52. [Crossref] [PubMed]
- Wang J, Hua H, Ran Y, et al. Derlin-1 is overexpressed in human breast carcinoma and protects cancer cells from endoplasmic reticulum stress-induced apoptosis. Breast Cancer Res 2008;10:R7. [Crossref] [PubMed]
- Akkoç Y, Berrak Ö, Arısan ED, et al. Inhibition of PI3K signaling triggered apoptotic potential of curcumin which is hindered by Bcl-2 through activation of autophagy in MCF-7 cells. Biomed Pharmacother 2015;71:161-71. [Crossref] [PubMed]
- Lee JJ, Loh K, Yap YS. PI3K/Akt/mTOR inhibitors in breast cancer. Cancer Biol Med 2015;12:342-54. [PubMed]
- Ellis MJ, Perou CM. The Genomic Landscape of Breast Cancer as a Therapeutic Roadmap. Cancer Discov 2013;3:27. [Crossref] [PubMed]
- Dong Q, Fu L, Zhao Y, et al. Derlin-1 is a target to improve radiotherapy effect of esophageal squamous cell carcinoma. Oncotarget 2017;8:55135-46. [Crossref] [PubMed]


