
Expression of SOX11 and HER2 and their association with recurrent breast cancer
IntroductionOther Section
Breast cancer is the most common form of cancer in women and the second leading cause of cancer-related deaths worldwide (1). Despite significant improvements in both early diagnosis and therapeutic interventions, clinical outcomes remain poor (2) due to significant heterogeneity in clinical, histological, and biological presentation (3,4). According to recent reports, 20% of patients who initially respond to therapeutic intervention will develop recurrent breast cancer within 10 years (5). Cancer cells that detach from primary breast cancer tissues can act as seed cells, resulting in metastatic cancer (6,7). These circulating tumor cells possess a variety of phenotypes similar to those of stem cells (8).
In most cases, breast cancer occurs as a result of mutations or dysregulation of important oncogenes, such as HER2, BRCA1, BRCA2, and PIK3CA (9-12). Sex determining region Y-box 11 (SOX11), initially discovered in 1995 (13), is 1 of 20 SOX genes identified to date that play vital roles in tissue remodeling, organ development, and neurogenesis (14,15). Overexpression of SOX11 has been reported in ovarian, brain, and mantle cell lymphoma (16-18), with SOX11 mutations resulting in significant dysregulation of downstream genes (19).
Human epidermal growth factor receptor 2 (HER2) is overexpressed in 25% of breast cancers, and is associated with high mortality and disease recurrence (20). Furthermore, HER2 overexpression is associated with cancer stem cell self-renewal, proliferation, and invasion, highlighting the importance of this gene in disease pathology (21). Here, we analyzed the expression of SOX11 and HER2 in recurrent breast cancer to better understand the association of these genes with disease outcomes.
MethodsOther Section
Experimental animals with recurrent breast cancer
Mouse experiments were performed using 6-month-old BALB/CJ female mice. All animals were carefully maintained under standard laboratory conditions, with all study-related protocols approved by our institution’s scientific review board. All mice were maintained in standard cages and provided with food and water ad libitum. For recurrent breast cancer, mice were injected with trypsinized 4T1 Luc2GFP cells (22) at two different dose ranges (~5,000 and 10,000 cells). After 1 week, mice were treated with doxorubicin (1 mg/kg weekly) and monitored for breast cancer recurrence. All animals subjected to experimental handling were observed regularly twice per day.
Histological imaging
Breast tissues were dissected and fixed in 10% neutral formalin solution. After 24 h, the tissues were washed thoroughly with distilled H2O and dried using a series of increasing concentrations of isopropyl alcohol (70% to 100% concentration). Finally, the tissues were rinsed with xylene and embedded in paraffin. Tissues were cut into thin 7-µm sections using a microtome and placed on glass slides. Tissue sections were then dewaxed and processed stepwise with xylene, isopropyl alcohol, and dH2O, and stained with hematoxylin and eosin.
Immunohistochemistry
After sectioning, the tissues were incubated with 3% H2O2 solution for 10 min, washed, and trypsinized for 5 min to unmask target antigens. Slides were then incubated with blocking solution [4% bovine serum albumin (BSA)] for 2 h at room temperature and incubated with primary antibodies (anti-SOX11, anti-HER2, or anti-ALDH1 antibodies; Abcam) at 4 °C for 8 h. Slides were then washed three times in 1× phosphate buffered saline (PBS) (2 min each), followed by treatment with secondary antibody at room temperature for 45 min. After washing, the slides were overlaid with a freshly prepared DAB solution, incubated at room temperature for 5 min, washed once in 1× PBS, and counterstained with hematoxylin.
Western blotting
Dissected tissue samples were mechanically lysed in ice-cold sample buffer. Cell lysates were then boiled for 10 min and stored at −80 °C until needed. Samples were loaded in equal concentrations (60 µg), resolved on 12% SDS-PAGE gel run at 50 V for 4 h, transferred to a PVDF membrane, and blocked in 5% non-fat milk for 1 h. The membrane was then incubated in primary antibody solution (anti-SOX11, anti-HER2, or anti-ALDH1 antibody; Abcam) at 4 °C overnight with gentle agitation. Blots were then washed, incubated with secondary antibody, and visualized with DAB solution.
Statistical analysis
All experiments were performed three or more times. Statistical analyses were performed using SPSS version 21.0, and the results expressed as the mean ± standard error. Comparisons between groups were performed using an ANOVA with Tukey’s post hoc test for multiple data comparison. P values <0.01 were considered statistically significant.
ResultsOther Section
Murine model of recurrent breast cancer
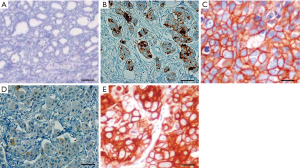
A murine model of mild and advanced stage recurrent breast cancer was developed using two groups of mice (n=5 per group) implanted with either low (5,000) or high (10,000) inocula of 4T1 Luc2GFP cells. After 1 week, mice were injected with 1 mg/kg doxorubicin weekly, with primary tumors excised on day 10. At 20 days post-implantation, mice inoculated with 5,000 cells exhibited signs of mild recurrent breast cancer, whereas the high inoculum group (10,000 cells) exhibited signs of advanced stage recurrent breast cancer. To better characterize tumor development, the primary tumors were subjected to histological analysis (Figure 1A,B,C,D,E). Both the high and low inoculum groups developed primary tumors with similar morphological features (Figure 1B,D); however, more divergent phenotypes were evident by day 20 (Figure 1C,E). The low inoculum group exhibited isolated clusters of cells with mild recurrent breast cancer (Figure 1C), whereas the high inoculum group harbored a larger number of proliferative cell clusters, consistent with that seen in advanced stage recurrent breast cancer (Figure 1E).

SOX11 expression in different stages of recurrent breast cancer
Expression of the transcription factor SOX11 is associated with tumor growth, progression, and invasion, with overexpression seen in a variety of cancers including basal-like breast cancer and ductal carcinoma (23,24). Here, we analyzed the expression of SOX11 in recurrent breast cancer induced by 4T1 Luc2GFP cell injection, along with their respective controls (Figure 2A,B,C,D,E). SOX11 was minimally expressed in normal breast tissue (Figure 2A), with modest increases seen in the primary tumors resected from the low inoculum group (Figure 2B). Following doxorubicin treatment, recurrent tumors exhibited a controlled pattern of SOX11 expression in the low inoculum group (Figure 2D). In contrast, mice in the high inoculum group exhibited stable overexpression of SOX11 (Figure 2C,E). Primary tumors excised on day 10 showed consistent overexpression of SOX11 around the cytoplasm (Figure 2C), whereas advanced stage recurrent breast cancers exhibited more pervasive expression of SOX11 that was evident throughout the cell (Figure 2E).
Controlled expression of HER2 upon treatment with doxorubicin
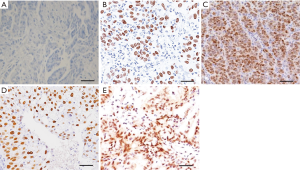
Overexpression of HER2 is often seen in recurrent breast cancer and is associated with more aggressive disease with shorter survival (20,25). Expression of HER2 was upregulated in a dose-dependent manner, with higher 4T1 Luc2GFP inocula exhibiting higher overall expression (Figure 3A,B,C). Upon treatment with doxorubicin, both mild (Figure 3D) and advanced stage (Figure 3E) recurrent cancers exhibited reduced HER2 expression. HER2 expression in recurrent cancer was consistent with that seen in primary tumors; however, the overall expression of HER2 was downregulated in these cells (Figure 3D).
Comparative analysis of SOX11, HER2, and ALDH1 expression
Western blotting was used to assess the relative expression of SOX11, HER2, and ALDH1, SOX11 and HER2 proteins (Figure 4) were expressed at levels similar to those observed by immunohistochemistry (Figures 2,3), with SOX11 expression consistently upregulated in aggressive recurrent breast cancer, even after treatment with doxorubicin (Figure 2E). To contextualize these findings, we next examined ALDH1 expression, a breast cancer stem cell marker (26), at different stages of recurrent breast cancer tissues. ALDH1 expression was strongly associated with SOX11 expression patterns, but not HER2 expression (Figure 4). Despite these differences in relative expression, elevated levels of SOX11, HER2, and ALDH1 were observed across breast cancer samples (Figure 5).


DiscussionOther Section
Breast epithelial cells of embryonic origin are composed of undifferentiated cells which differentiate after birth and give rise to several different breast epithelial cell populations (27). These stem cell-like cells behave as cancer stem cells in many solid tumors and support the growth of cancer cells even after treatment with various chemotherapeutic regimens (28). Here, we developed a mouse model of recurrent breast cancer using 4T1 Luc2GFP cells. Histological studies showed that mild forms of recurrent breast cancer, in conjunction with the primary tumors excised from these animals, are readily controlled with doxorubicin therapy, whereas more advanced stages of recurrent breast cancer remain resistant to therapeutic intervention. These result suggests that the risk of death in early stage recurrent breast cancer is not correlated with the overall spectrum of breast cancer mortality (29).
Histological analysis of SOX11 expression in advanced stage recurrent breast cancer revealed consistent overexpression throughout the cell, consistent with the invasive nature and progression of breast cancer (Figure 1C,E; Figure 2C,E). HER2-positive cells are also a sign of aggressive cancer, representing a significant risk for tumor recurrence. Treatment with doxorubicin was found to reduce HER2 expression in mild and advanced stage recurrent breast cancer (Figure 3A,B,C,D,E), whereas both ALDH1 and SOX11 were consistently overexpressed in both tumor types (Figure 4). These results suggest that cancer progression may be attenuated in response to treatment, as evidenced by the reduction in HER2 expression, but these effects do not extend to breast cancer stem cells, which continue to exhibit high levels of SOX11 expression. This comparative analysis showed that SOX11 and ALDH1 are better markers for breast cancer stem cells than HER2, and may be predictive of cancer recurrence.
Several studies have identified HER2 as an important regulator of breast cancer stem cells, as inhibition of HER2 expression reduces cancer stem cell populations (21). However, our data indicated that HER2 expression levels were not associated with breast cancer stem cell survival in recurrent breast cancers. These data suggest that HER2 expression is an effective marker for assessing treatment response, but is not appropriate for predicting responses of breast cancer stem cells.
ConclusionsOther Section
In summary, using 4T1 Luc2GFP cells, we established effective mouse models of mild and advanced stage recurrent breast cancer. SOX11 expression was associated with breast cancer stem cell survival and was strongly correlated with ALDH1 expression. In contrast, HER2 expression was associated with treatment response in recurrent breast cancer but was not predictive of changes associated with breast cancer stem cells.
AcknowledgmentsOther Section
Funding: None.
FootnoteOther Section
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.01.27). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All animals were carefully maintained under standard laboratory conditions, with all study-related protocols approved by our institution’s scientific review board (approval No. TXT24311).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- Sun YS, Zhao Z, Yang ZN, et al. Risk Factors and Preventions of Breast Cancer. Int J Biol Sci 2017;13:1387-97. [Crossref] [PubMed]
- Bodai BI, Tuso P. Breast cancer survivorship: a comprehensive review of long-term medical issues and lifestyle recommendations. Perm J 2015;19:48-79. [Crossref] [PubMed]
- Vargo-Gogola T, Rosen JM. Modelling breast cancer: one size does not fit all. Nat Rev Cancer 2007;7:659-72. [Crossref] [PubMed]
- Weigelt B, Reis-Filho JS. Histological and molecular types of breast cancer: is there a unifying taxonomy? Nat Rev Clin Oncol 2009;6:718-730. [Crossref] [PubMed]
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2008, National Cancer Institute. Bethesda, MD, 2010.
- Alix-Panabières C, Riethdorf S, Pantel K. Circulating tumor cells and bone marrow micrometastasis. Clin Cancer Res 2008;14:5013-21. [Crossref] [PubMed]
- Kim MY, Oskarsson T, Acharyya S, et al. Tumor self-seeding by circulating cancer cells. Cell 2009;139:1315-26. [Crossref] [PubMed]
- Kasimir-Bauer S, Hoffmann O, Wallwiener D, et al. Expression of stem cell and epithelial-mesenchymal transition markers in primary breast cancer patients with circulating tumor cells. Breast Cancer Res 2012;14:R15. [Crossref] [PubMed]
- Bartlett JM, Going JJ, Mallon EA, et al. Evaluating HER2 amplification and overexpression in breast cancer. J Pathol 2001;195:422-8. [Crossref] [PubMed]
- Miki Y, Swensen J, Shattuck-Eidens D, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science 1994;266:66-71. [Crossref] [PubMed]
- Easton DF, Steele L, Fields P, et al. Cancer risks in two large breast cancer families linked to BRCA2 on chromosome 13q12-13. Am J Hum Genet 1997;61:120-8. [Crossref] [PubMed]
- Campbell IG, Russell SE, Choong DY, et al. Mutation of the PIK3CA gene in ovarian and breast cancer. Cancer Res 2004;64:7678-81. [Crossref] [PubMed]
- Jay P, Goze C, Marsollier C, et al. The human SOX11 gene: cloning, chromosomal assignment and tissue expression. Genomics 1995;29:541-5. [Crossref] [PubMed]
- Bhattaram P, Penzo-Méndez A, Sock E, et al. Organogenesis relies on SoxC transcription factors for the survival of neural and mesenchymal progenitors. Nat Commun 2010;1:9. [Crossref] [PubMed]
- Haslinger A, Schwarz TJ, Covic M, et al. Expression of Sox11 in adult neurogenic niches suggests a stage-specific role in adult neurogenesis. Eur J Neurosci 2009;29:2103-14. [Crossref] [PubMed]
- Brennan DJ, Ek S, Doyle E, et al. The transcription factor Sox11 is a prognostic factor for improved recurrence-free survival in epithelial ovarian cancer. Eur J Cancer 2009;45:1510-7. [Crossref] [PubMed]
- Weigle B, Ebner R, Temme A, et al. Highly specific overexpression of the transcription factor SOX11 in human malignant gliomas. Oncol Rep 2005;13:139-44. [PubMed]
- Dictor M, Ek S, Sundberg M, et al. Strong lymphoid nuclear expression of SOX11 transcription factor defines lymphoblastic neoplasms, mantle cell lymphoma and Burkitt’s lymphoma. Haematologica 2009;94:1563-8. [Crossref] [PubMed]
- Wang X, Björklund S, Wasik AM, et al. Gene expression profiling and chromatin immunoprecipitation identify DBN1, SETMAR and HIG2 as direct targets of SOX11 in mantle cell lymphoma. PLoS One 2010;5:e14085. [Crossref] [PubMed]
- Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987;235:177-82. [Crossref] [PubMed]
- Korkaya H, Paulson A, Iovino F, et al. HER2 regulates the mammary stem/progenitor cell population driving tumorigenesis and invasion. Oncogene 2008;27:6120-30. [Crossref] [PubMed]
- Bailey-Downs LC, Thorpe JE, Disch BC, et al. Development and characterization of a preclinical model of breast cancer lung micrometastatic to macrometastatic progression. PLoS One 2014;9:e98624. [Crossref] [PubMed]
- Shepherd JH, Uray IP, Mazumdar A, et al. The SOX11 transcription factor is a critical regulator of basal-like breast cancer growth, invasion, and basal-like gene expression. Oncotarget 2016;7:13106-21. [Crossref] [PubMed]
- Oliemuller E, Kogata N, Bland P, et al. SOX11 promotes invasive growth and DCIS progression. J Pathol 2017;243:193-207. [Crossref] [PubMed]
- Meric-Bernstam F, Hung MC. Advances in targeting human epidermal growth factor receptor-2 signaling for cancer therapy. Clin Cancer Res 2006;12:6326-30. [Crossref] [PubMed]
- de Beça FF, Caetano P, Gerhard R, et al. Cancer stem cells markers CD44, CD24 and ALDH1 in breast cancer special histological types. J Clin Pathol 2013;66:187-91. [Crossref] [PubMed]
- Howard BA, Veltmaat JM. Embryonic mammary gland development; a domain of fundamental research with high relevance for breast cancer research. J Mammary Gland Biol Neoplasia 2013;18:89-91. [Crossref] [PubMed]
- Nguyen LV, Vanner R, Dirks P, et al. Cancer stem cells: an evolving concept. Nat Rev Cancer 2012;12:133-43. [Crossref] [PubMed]
- Sopik V, Nofech-Mozes S, Sun P, et al. The relationship between local recurrence and death in early-stage breast cancer. Breast Cancer Res Treat 2016;155:175-85. [Crossref] [PubMed]


