
Good response to neoadjuvant chemoradiotherapy predicts good oncological outcome in locally advanced rectal cancer
Introduction
Neoadjuvant chemoradiotherapy (CRT) followed by total mesorectal excision has become the standard treatment for patients with locally advanced rectal cancer (LARC). Neoadjuvant CRT is related to preferable locoregional control and lower therapy compliance compared to postoperative CRT (1). Nowadays, most authorities have recommended the form of concurrent long-course CRT (2-4), either concurrent with 5-fluorouracil (5-FU) or Capecitabine (5), especially in patients with threatened or involved mesorectal fascia (MRF), tumor with adjacent organ invasion or low position tumor. The tumor pathological response to the CRT is evaluated by tumor regression grade (TRG) (6), which is a semi-quantitative scoring of the relative proportion of residual tumor to stromal fibrosis. Several studies have reported a higher rate of pathological complete response (pCR) of tumor (10–30%) using neoadjuvant chemotherapy with radiation (7,8). Patients who have achieved a pCR are associated with an improved long-term outcome (9,10). Habr-Gama (11) has supported a non-operative policy “watch and wait” in those patients with clinical complete response whose local failure rate was reported only 3% (12). What’s more, a near pCR, moderate response (TRG1), can possibly translate into much better clinical outcomes after surgery compared to those who remain massive residual tumor in the resected specimens. The aim of this retrospective study was to investigate the predictors of clinicopathologic parameters and treatment-related variables on tumor regression grade, disease-free survival (DFS), and overall survival (OS).
Methods
Patients
The retrospective study enrolled consecutive patients undergoing preoperative CRT and curative resection from March 2014 to December 2017 at People’s hospital of Jiangsu Province, People’s Republic of China. Tumor stage was evaluated using a combination of colonoscopy, chest and abdominal enhanced CT, and pelvic enhanced MRI before CRT, according to 7th American Joint Committee on Cancer staging system (AJCC) TNM staging system. The enrolled patients met the inclusion criteria: (I) patho-histologically confirmed adenocarcinoma; (II) T3–4 or N+ disease in initially; (III) received preoperative CRT followed by radical resection. Patients were excluded if they harbored metastatic disease before or during preoperative treatment or died within 1 month postoperatively. The demographics, preoperative treatment, primary tumor characteristics, and follow-ups were reviewed in details.
This study was approved by the Ethics Committee of Jiangsu Province People’s Hospital, in accordance by the Helsinki Declaration. Written informed consent was required for participation.
Treatment
A total of 50.0–50.4 Gy was delivered in 1.8–2.0 Gy per daily fractions to the pelvic area without tumor gross bonus. Concurrent chemotherapy regiments were classified into: Capecitabine 825 mg/m2 bid D1–5 qw; Oxaliplatin 50 mg/m2/qw + Capecitabine 625 mg/m2 bid D1–5 qw; CPT-11 80 mg/m2/qw + Capecitabine 625 mg/m2 bid D1–5 qw. Radical surgical resection was conducted several weeks after completing CRT. The TME surgery procedure was mainly including Dixon, Miles, Hartmann, and so on. The resected specimens were reviewed by two experienced pathologists. The ypT stages of specimens and the tumor regression grade were evaluated on the basis of the 7th AJCC TNM staging system (13): (I) complete response (TRG score 0), no viable cancer cells; (II) moderate response: (TRG score 1), single cells or small groups of cancer cells; (III) minimal response: (TRG score 2), residual cancer outgrown by fibrosis and; (IV) poor response: (TRG score 3), minimal or no tumor kill.
Follow-up
Patients were either reviewed in outpatients or contacted by telephone every three months for the first 2 years after surgery, and every 6 months for the next 3 years. Chest, abdominal or pelvic CT, and pelvic MRI were performed every 6 months, and colonoscopy was conducted annually during follow-up. Overall survival was defined as the interval from surgery to the date of death or last follow-up, and DFS was defined as the time from surgery to the date of disease recurrence or last follow-up. Follow-up statistics were reviewed by February 28, 2018.
Statistical analysis
The statistical analysis was performed by the IBM SPSS Statistics 22.0 software (Chicago, IL, USA) and GraphPad Prism 5 software (San Diego, CA, USA). Continuous variables were evaluated by the parametric Student’s t-test, while categorical variables were compared by the Chi-square test or Fisher’s exact test. DFS and OS were calculated in the Kaplan-Meier model and comparisons were analyzed by Cox regression analysis. Variables that were significant in univariate analysis were applied to multivariate Cox regression analysis. Two-tailed P≤0.05 was considered statistically significant.
Results
Patient demographics
One hundred and forty-one LARC patients were accepted neo-adjuvant therapy, while 5 patients failed to operate due to extensive peritoneal or visceral metastasis, and 4 patients were only conducted palliative colostomy for local gross tumor. One hundred and thirty-two individuals finally proceed to surgical operation, 62.1% (82/132) were males and 37.9% (50/132) were females. 95.5% of patients were T3–4 stage and 89.3% of patients were node positive. The median age was 58 years (range from 22 to 80), and the median interval days from the end of radiotherapy to surgery was 61 days (range from 29 to 122). Forty-four patients underwent Miles operation, 6 patients underwent Hartmann procedure and 82 patients were received Dixons, of whom 26 patients were given colostomy in preventing anastomotic leakage. Median follow-up time was 21.5 months (range from 1 to 38). Baseline characteristics are listed in Table 1.
Table 1
| Clinical characteristics | Total | Complete response | Good response | |||||
|---|---|---|---|---|---|---|---|---|
| pCR | non-pCR | P | TRG0-1 | TRG2-3 | P | |||
| Gender | 0.001* | 0.199 | ||||||
| Female | 50 | 18 | 32 | 24 | 26 | |||
| Male | 82 | 8 | 74 | 32 | 50 | |||
| Age (year, median) | 58 [22–80] | |||||||
| T stage before CRT | 0.566 | 0.789 | ||||||
| cT2 | 6 | 2 | 4 | 2 | 4 | |||
| cT3 | 76 | 14 | 62 | 34 | 42 | |||
| cT4 | 50 | 10 | 40 | 20 | 30 | |||
| N stage before CRT | 0.858 | 0.967 | ||||||
| cN0 | 14 | 2 | 12 | 6 | 8 | |||
| cN1 | 54 | 10 | 44 | 22 | 32 | |||
| cN2 | 64 | 14 | 50 | 28 | 36 | |||
| Anal edge distance | 2–11 cm | |||||||
| Pathological pattern | 0.807 | 0.967 | ||||||
| Adenocarcinoma | 98 | 20 | 78 | 48 | 50 | |||
| Mucinous | 34 | 6 | 28 | 8 | 26 | |||
| Circumferential involvement | 0.632 | 0.325 | ||||||
| ≤1/2 | 36 | 8 | 28 | 18 | 18 | |||
| ﹥1/2 | 96 | 18 | 78 | 38 | 58 | |||
| Chemotherapy | 0.148 | 0.048* | ||||||
| Single-agent | 36 | 4 | 32 | 10 | 26 | |||
| Dual-agents | 96 | 22 | 74 | 46 | 50 | |||
| Interval (day, median) CRT to surgery | 61 [29–122] | 0.643 | 0.709 | |||||
| ≤8 weeks | 44 | 10 | 34 | 20 | 24 | |||
| ﹥8 weeks | 88 | 16 | 72 | 36 | 52 | |||
| Operative procedure | 0.746 | 0.005* | ||||||
| Miles | 44 | 10 | 34 | 20 | 24 | |||
| Dixon | 56 | 10 | 46 | 30 | 26 | |||
| Dixon with preventive colostomy | 26 | 6 | 20 | 6 | 20 | |||
| Hartmann | 6 | 0 | 6 | 0 | 6 | |||
*, P﹤0.05. CRT, chemoradiotherapy; TRG, tumor regression grade.
Factors associated with pathologic response
Among the patients 19.7% (26/132) had gotten pCR (TRG0) and 19.7% (26/132) had gotten moderate response (TRG 1). Fifty-six point one percent (74/132) patients was observed with minimal response (TRG 2) and 4.5% (6/132) patients were diagnosed with response (TRG 3). Eight patients with the pCR and twenty with the TRG1 had a primary cT4 tumor, which were both classified as good response. Of note: 12 of 26 (46.2%) pCR patients previously had mesorectal lymph node metastases. No pre-treatment factors (age, cT, cN, type of pathology) but gender was significantly associated with pCR (P=0.001). Among treatment factors, good response occurred more frequently in those treated with combined dual-agent chemotherapy (P=0.048).
Toxicity
The combined incidence of grade 3–4 acute radiotherapy toxicity to the skin and bowel toxicity was 9.1%. In terms of grade 3 or above late radiotherapy toxicity to the bowel, and urinary tract was 10.6%. For grade 3 or above myelosuppression, the incidences of neutropenia, anemia, and thrombocytopenia were 10.6%, 2.27%, and 1.5%, respectively. The most common non-hematological grade 3 or above acute toxicity was diarrhea (5.7%). With regard to surgical complications, there were 10 patients (7.6%) with delayed wound healing, 10 patients (7.6%) with anastomotic leakages, 4 patients (3.0%) with anastomotic stenosis, and 3 patients (2.3%) with post-operative ileus. Thirty-day postoperative mortality was barely reported.
Survival
With a median follow-up period of 21.5 months, 107 (81.1%) patients were being alive, and 100 patients (75.8%) were free of recurrence or metastasis, and others (5.3%) suffered from distant metastasis and underwent anti-cancer therapy. Twenty-two patients (16.7%) were died of tumor progress (3 local recurrence only), and 5 patients (3.8%) were died of other causes. Univariate analyses for OS have shown that cN2, pathologic pattern, operative procedure and tumor regression grade were statistically significant, but after multivariate adjustment, only TRG remained significant (HR =0.306, 95% CI: 0.096–0.976, P=0.045) (Table 2 and Figure 1 shown). Univariate analysis revealed that pathological T stage, and TRG could potentially influence DFS, but only TRG (HR =0.257, 95% CI: 0.103–0.643, P=0.004) remained significant after multivariate adjustment (Table 2 and Figure 1D shown). There was no significant DFS and OS between pCR and non-pCR in (Figure 1A,C shown). We also compared the OS and DFS between TRG0, TRG1 and poor response (TRG2/3). The DFS between TRG1 and TRG2/3 was statistically significant (HR =0.397, 95% CI: 0.159–0.993, P=0.048), but not in OS. No significant differences in OS and DFS between TRG0 and TRG1 (Table 3 shown).
Table 2
| Clinical characteristics | OS | DFS | |||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| Univariate | |||||
| Gender | |||||
| Female | 0.563 (0.220–1.440) | 0.231 | 0.940 (0.459–1.926) | 0.867 | |
| Male | 1.00 | 1.00 | |||
| Age | 1.004 (0.948–1.042) | 0.822 | 0.988 (0.960–1.017) | 0.413 | |
| T stage before CRT | |||||
| cT2 | 1.00 | 1.00 | |||
| cT3 | 1.498 (0.308–7.286) | 0.616 | 2.015 (0.641–6.336) | 0.231 | |
| cT4 | 0.874 (0.356–2.147) | 0.769 | 0.851 (0.402–1.803) | 0.674 | |
| N stage before CRT | |||||
| cN0 | 1.00 | 1.00 | |||
| cN1 | 0.350 (0.078–1.564) | 0.169 | 0.903 (0.300–2.719) | 0.857 | |
| cN2 | 0.278 (0.103–0.752) | 0.012* | 0.685 (0.321–1.464) | 0.329 | |
| Pathological pattern | |||||
| Adenocarcinoma | 0.353 (0.152–0.819) | 0.015* | 0.494 (0.241–1.012) | 0.054 | |
| Mucinous | 1.00 | 1.00 | |||
| Circumferential involvement | |||||
| ≤1/2 | 0.562 (0.189–1.668) | 0.299 | 1.231 (0.582–2.605) | 0.587 | |
| ﹥1/2 | 1.00 | 1.00 | |||
| Interval (CRT to surgery) | |||||
| ≤8 weeks | 0.730 (0.289–1.841) | 0.505 | 0.962 (0.461–2.007) | 0.918 | |
| ﹥8 weeks | 1.00 | 1.00 | |||
| Chemotherapy | |||||
| Single agent | 1.325 (0.506–3.472) | 0.567 | 1.304 (0.615–2.766) | 0.489 | |
| Dual agents | 1.00 | 1.00 | |||
| Operative procedure | |||||
| Miles | 1.00 | 1.00 | |||
| Dixon | 0.171 (0.034–0.870) | 0.033* | 0.538 (0.121–2.392) | 0.416 | |
| Hartmann | 0.198 (0.043–0.908) | 0.037* | 0.330 (0.075–1.451) | 0.142 | |
| Pathological response | |||||
| pCR | 0.388 (0.090–1.664) | 0.202 | 0.493 (0.173–1.407) | 0.186 | |
| non-pCR | 1.00 | 1.00 | |||
| Tumor regression | |||||
| TRG0–1 | 0.226 (0.077–0.670) | 0.007* | 0.229 (0.094–0.558) | 0.001* | |
| TRG2–3 | 1.00 | 1.00 | |||
| N stage after CRT | |||||
| pN0 | 1.00 | 1.00 | |||
| pN1 | 0.895 (0.201–3.978) | 0.884 | 0.224 (0.087–0.581) | 0.002* | |
| pN2 | 1.262 (0.229–6.951) | 0.789 | 0.941 (0.338–2.622) | 0.907 | |
| Multivariable | |||||
| Tumor regression | 0.306 (0.096–0.976) | 0.045* | 0.257 (0.103–0.643) | 0.004* | |
| N stage before CRT | 0.411 (0.142–1.192) | 0.102 | 0.536 (0.212–1.355) | 0.188 | |
| Pathological pattern | 0.620 (0.230–1.672) | 0.345 | – | – | |
| Operative procedure | – | – | |||
| Miles | 1.00 | ||||
| Dixon | 0.266 (0.044–1.625) | 0.152 | |||
| Hartmann | 0.394 (0.075–2.065) | 0.270 | |||
*, P﹤0.05. CRT, chemoradiotherapy.
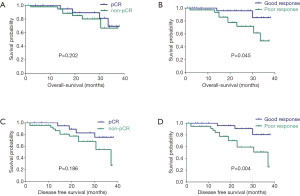
Table 3
| Tumor regression | OS | DFS | |||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| TRG0 vs. TRG1 | |||||
| TRG0 | 1.118 (0.157–7.961) | 0.911 | 0.953 (0.134–6.772) | 0.962 | |
| TRG1 | 1.00 | 1.00 | |||
| TRG1 vs. TRG2-3 | |||||
| TRG1 | 0.476 (0.181–1.250) | 0.132 | 0.397 (0.159–0.993) | 0.048* | |
| TRG2-3 | 1.00 | 1.00 | |||
| TRG0 vs. TRG2-3 | |||||
| TRG0 | 0.024 (0.00–3,742.4) | 0.540 | 0.582 (0.00–9,275.2) | 0.582 | |
| TRG2-3 | 1.00 | 1.00 | |||
*, P﹤0.05.
Discussion
Short-term therapeutic efficacy
Neo-adjuvant CRT followed by radical surgery is the current standards for local advanced rectal cancer. The combination of chemotherapy and radiation has been proved to further improve tumor downstage in an effort to increase surgical resection and the pCR rate more than with preoperative radiation alone. Recent studies have shown the pCR rate range from 14% to 28% (14-18), and our study got a similar pCR rate of 19.7%. We also discovered a same proportion about 19.7% of near pCR (TRG1). These patients with better tumor regression can obtain an effort to maximize surgical resection of previous marginal and unresectable tumors, which can effectively reduce the local recurrence.
In the clinic, post-CRT MRI is routinely used to investigate the tumor regression compared with pre-CRT MRI by Dworak’s standard. Cui Y has utilized pre-treatment radiomics analysis of multiparametric MRI for prediction of pCR after neoadjuvant CRT (19). However, we still encounter over-stage in post-CRT MRI. It has been suggested that MRI is often difficult to differentiate between viable tumor, residual fibrotic non-tumor tissue, and desmoplastic reaction, resulting in poor agreement between MRI staging and pathologic staging in both T and N stages (20). To avoid the bias we evaluated tumor regression grade according to 7th AJCC neo-adjuvant pathologic stage.
Numerous retrospective studies have previously identified various disease-related variables as potential predictors of pCR, which included tumor size, pre-treatment T/N category, cytotoxic therapy, low tumor grade and so on. In our study we only found significance between gender and pCR with limited clinical and pathologic data.
Only few patients can achieve a pCR, a strict pathologic remission. We found that some patients with near pCR (TRG1) had a survival and local control approximate to those with a pCR (21-23). Conversely, some papers have reported these patients with near pCR had unexpectedly poor outcomes (DFS or OS) and harbored nodal metastases, which was corresponding to those poor responders at all (24,25). In our study, we classified TRG0-1 with ypN0 as good responder, and discovered dual-drug chemotherapy related to better tumor shrinkage. But no difference was found between the two dual-agent regimens. It has been observed that combinations such as 5-FU/Oxaliplatin or Irinotecan/5-FU have higher response rates than single agents such as 5-FU alone (26), which consists with our results. While increased gastrointestinal, mucosal, and hematologic toxicity were observed in the dual-drug group, especially grade 3 or above of neutropenia and acute diarrhea. It was recently reported a retrospective cohort study based on 2,094 patients that lengthening the interval (>13 weeks) from CRT to surgery improves the pathological response (27). However, we didn’t find a longer interval translated into an increase in pCR (TRG0) or near pCR (TRG1) rate. It was considered that insufficient number of cases and a higher proportion of N2 patients in the long interval group weak the tumor shrinkage. However, we observed higher surgical morbidity about delayed incision healing and anastomotic haemorrhage in shorter interval group due to radiotherapy induced tissue swelling and inflammation (28,29). Furthermore, surgeons found soft tissue fibrosis and friability in most of patients after neoadjuvant CRT, while the fibrosis did not translate into a significantly increased technical difficulty of operation or postoperative complications.
Long-term survival
The median follow-up time is 21.5 months, as most patients with progressive disease suffered from distance metastasis rather than local relapse. Most recurrences occurred within the first 2 years, and distant metastasis became the dominating outcome, which developed up to 73.3% in progressive patients. Although pathologic pattern, N stage before CRT, tumor regression, operative procedure and N stage after CRT were shown an association with DFS or OS, only tumor regression grade was a potential factor for OS and DFS after multivariate adjustment.
Tumor regression grade has been implemented to predict oncologic outcomes in many articles with inconsistent results, probably due to the lack of uniform pathologic response evaluation or definitions for TRG. A systematic review has demonstrated the prognostic value of TRG in predicting long-term survival (DFS and OS) (30).
In our study patients with pCR having 1-year DFS of 100%, compared to those with non-pCR of 89.5%. These results reflect an outcome similar to that in other studies where patients having pCR have excellent outcomes (31). The 1-year DFS were 100% in patients with good response (TRG0-1), compared to those with poor response (TRG2-3) of 88.0%, which supported the fact that TRG 0-1 own the similar survival and local control to those with a pCR. We postulated that our data reflected prognosis more accurately in patients with T3 with risk factors, T4 and/or N2 disease, treated with neoadjuvant CRT. Also, our cohort contained patients with advanced diseases, the inclusion criteria is also the reason for the different survival figures. However, our subgroup with a pCR unexpectedly could not predict statistically better long-term outcomes. It was retrospectively found two patients with pCR had distant metastasis in the short term and died soon (14 and 13 months after surgery), which exceptionally effected the long-term survival of pCR group. The possible reasons were that both patients might already have had simultaneous metastasis before CRT with insufficient intensity of chemotherapy regimen and the tumor was sensitive to radiation but strongly invasive and metastatic. And the follow-up time is limited, the survival advantage of pCR may be not yet reflected. TRG1 has better local and remote control in DFS compared with TRG2-3, leading to the non-pCR group has obtained a better outcome. It strongly supported that TRG1 could demonstrate an excellent local and remote control among this partial non-pCR group.
Limits in our study are obvious and need to be improved, including lack of postoperative CRM status, deficiency of follow-up time and missing information of adjuvant chemotherapy. We lack CRM status in some of our pathological specimens, which has been shown an independent prognostic factor, predicting local recurrence, distant metastasis and OS (32). The long-term result from EORTC 22921 and similar studies showed that adjuvant chemotherapy after preoperative radiotherapy did not affect DFS or OS in cT3–4 resectable rectal cancer (33). However, studies have shown that adding oxaliplatin to adjuvant and/or neo-adjuvant treatment can improve DFS (34,35). In our study, we didn’t mention the relationship between adjuvant chemotherapy and survival for the insufficient follow-up.
In conclusion, neoadjuvant CRT for LARC patients is effective and leads to an acceptable outcome. Tumor regression after CRT is the most significant prognostic factor in OS and DFS, after multivariate adjustment. Pathologic assessment of tumor regression, better tumor regression (TRG0-1) after CRT can also be used to predict the oncologic outcomes amongst other factors.
Acknowledgments
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.01.17). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Ethics Committee of Jiangsu Province People’s Hospital, in accordance by the Helsinki Declaration (as revised in 2013). Written informed consent was required for participation.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fleming FJ, Påhlman L, Monson JR. Neoadjuvant therapy in rectal cancer. Dis Colon Rectum 2011;54:901-12. [Crossref] [PubMed]
- Glimelius B, Tiret E, Cervantes A, et al. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24:vi81-8. [Crossref] [PubMed]
- Minsky BD. Counterpoint: long-course chemoradiation is pref- erable in the neoadjuvant treatment of rectal cancer. Semin Radiat Oncol 2011;21:228-33. [Crossref] [PubMed]
- Moureau-Zabotto L, Farnault B, de Chaisemartin C, et al. Predictive factors of tumor response after neoadjuvant chemoradiation for locally advanced rectal cancer. Int J Radiat Oncol Biol Phys 2011;80:483-91. [Crossref] [PubMed]
- Hofheinz RD, Wenz F, Post S, et al. Chemoradiotherapy with capecitabine versus fluorouracil for locally advanced rectal cancer: a rand- omised, multicentre, non-inferiority, phase 3 trial. Lancet Oncol 2012;13:579-88. [Crossref] [PubMed]
- Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis 1997;12:19-23. [Crossref] [PubMed]
- Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731-40. [Crossref] [PubMed]
- Bosset JF, Calais G, Mineur L, et al. Does the addition of chemo- therapy (CT) to preoperative radiotherapy (preopRT) increase the pathological response in patients with resected rectal cancer: Report of the 22921 EORTC phase III trial. J Clin Oncol 2004;23:247.
- Maas M, Nelemans PJ, Valentini V, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: A pooled analysis of individual patient data. Lancet Oncol 2010;11:835-44. [Crossref] [PubMed]
- Capirci C, Valentini V, Cionini L, et al. Prognostic value of pathologic complete response after neoadjuvant therapy in locally advanced rectal cancer: Long-term analysis of 566 ypCR patients. Int J Radiat Oncol Biol Phys 2008;72:99-107. [Crossref] [PubMed]
- Habr-Gama A, Perez RO, Nadalin W, et al. Long-term results of preoperative chemoradiation for distal rectal cancer correlation between final stage and survival. J Gastrointest Surg 2005;9:90-9. [Crossref] [PubMed]
- Habr-Gama A, Gama-Rodrigues J, São Julião GP, et al. Local recurrence after complete clinical response and watch and wait in rectal cancer after neoadjuvant chemoradiation: impact of salvage therapy on local disease control. Int J Radiat Oncol Biol Phys 2014;88:822-8. [Crossref] [PubMed]
- Edge S, Byrd DR, Compton CC, et al. editors. AJCC cancer staging handbook: from the AJCC cancer staging manual. 7th edition. Vol. 2010. New York: Springer, 2010.
- Gérard JP, Azria D, Gourgou-Bourgade S, et al. Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: Results of the phase III trial ACCORD 12/0405-Prodige 2. J Clin Oncol 2010;28:1638-44. [Crossref] [PubMed]
- Aschele C, Cionini L, Loardi S, et al. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: Pathologic results of the STAR-01 randomized phase III trial. J Clin Oncol 2011;29:2773-80. [Crossref] [PubMed]
- Roh MS, Colangelo LH, O’Connell MJ, et al. Preoperative multi-modality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol 2009;27:5124-30. [Crossref] [PubMed]
- Roh MS, Yothers GA, O’Connell MJ, et al. The impact of capecitabine and oxaliplatin in the preoperative multimodality treatment in patients with carcinoma of the rectum: NSABP R-04. J Clin Oncol 2011;29. abstract 3503.
- Wong SJ, Winter K, Meropol NJ, et al. RTOG 0247: A randomized phase II study of neoadjuvant capecitabine and irinotecan versus capecitabine and oxaliplatin with concurrent radiation therapy for Patients with locally advanced rectal cancer. Int J Radiat Oncol Biol Phys 2012;82:1367-75. [Crossref] [PubMed]
- Cui Y, Yang X, Shi Z, et al. Radiomics analysis of multiparametric MRI for prediction of pathological complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Eur Radiol 2019;29:1211-20. [Crossref] [PubMed]
- Chen CC, Lee RC, Lin JK, et al. How accurate is magnetic resonance imaging in restaging rectal cancer in patients receiving preoperative combined chemoradiotherapy? Dis Colon Rectum 2005;48:722-8. [Crossref] [PubMed]
- Vecchio FM, Valentini V, Minsky BD, et al. The relationship of pathologic tumor regression grade (TRG) and outcomes after preoperative therapy in rectal cancer. Int J Radiat Oncol Biol Phys 2005;62:752-60. [Crossref] [PubMed]
- Beddy D, Hyland JM, Winter DC, et al. A simplified tumor regression grade correlates with survival in locally advanced rectal carcinoma treated with neoadjuvant chemoradiotherapy. Ann Surg Oncol 2008;15:3471-7. [Crossref] [PubMed]
- Guillem JG, Chessin DB, Cohen AM, et al. Long-term oncologic outcome following preoperative combined modality therapy and total mesorectal excision of locally advanced rectal cancer. Ann Surg 2005;241:829-36. [Crossref] [PubMed]
- Gosens MJ, Klaassen RA, Tan-Go I, et al. Circumferential margin involvement is the crucial prognostic factor after multimodality treatment in patients with locally advanced rectal carcinoma. Clin Cancer Res 2007;13:6617-23. [Crossref] [PubMed]
- Rödel C, Martus P, Papadoupolos T, et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol 2005;23:8688-96. [Crossref] [PubMed]
- Mitchell EP. Irinotecan in preoperative combined modality therapy for locally advanced rectal cancer. Oncology 2000;14:56-9. [PubMed]
- Macchia G, Gambacorta MA, Masciocchi C, et al. Time to surgery and pathologic complete response after neoadjuvant chemoradiation in rectal cancer: A population study on 2094 patients. Clin Transl Radiat Oncol 2017;4:8-14. [Crossref] [PubMed]
- Wolthuis AM, Penninckx F, Haustermans K, et al. Impact of interval between neoadjuvant chemoradiotherapy and TME for locally advanced rectal cancer on pathologic response and oncologic outcome. Ann Surg Oncol 2012;19:2833-41. [Crossref] [PubMed]
- Tran CL, Udani S, Holt A, et al. Evaluation of safety of increased time interval between chemoradiation and resection for rectal cancer. Am J Surg 2006;192:873-7. [Crossref] [PubMed]
- Kong JC, Guerra GR, Warrier SK, et al. Prognostic value of tumour regression grade in locally advanced rectal cancer: a systematic review and meta-analysis. Colorectal Dis 2018;20:574-85. [Crossref] [PubMed]
- Maas M, Nelemans PJ, Valentini V, et al. Long-term outcome in patients with a pathologic complete response after chemoradiation for rectal cancer: a pooled analysis of 3105 patients. Lancet Oncol 2010;11:835-844. [Crossref] [PubMed]
- Wibe A, Rendedal PR, Svensson E, et al. Prognostic significance of the circumferential resection margin following total mesorectal excision for rectal cancer. Br J Surg 2002;89:327-34. [Crossref] [PubMed]
- Sainato A, Cernusco Luna Nunzia V, Valentini V, et al. No benefit of adjuvant Fluorouracil Leucovorin chemotherapy after neoadjuvant chemo- radiotherapy in locally advanced cancer of the rectum (LARC): Long term results of a randomized trial (I-CNR-RT). Radiother Oncol 2014;113:223-9. [Crossref] [PubMed]
- Hong YS, Nam BH, Kim KP, et al. Oxaliplatin, fluorouracil, and leucovorin versus fluorouracil and leucovorin as adjuvant chemotherapy for locally advanced rectal cancer after preoperative chemoradiotherapy (ADORE): an open-label, multicentre, phase 2, randomised controlled trial. Lancet Oncol 2014;15:1245-53. [Crossref] [PubMed]
- Rödel C, Graeven U, Fietkau R, et al. Oxaliplatin added to fluorouracil-based pre- operative chemoradiotherapy and postoperative chemo- therapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open- label, randomised, phase 3 trial. Lancet Oncol 2015;16:979-89. [Crossref] [PubMed]


