Intensive patient’s care program reduces anxiety and depression as well as improves overall survival in de novo acute myelocytic leukemia patients who underwent chemotherapy: a randomized, controlled study
Introduction
Acute myeloid leukemia (AML) is a malignant hematopoietic disorder characterized by the clonal expansion of undifferentiated myeloid precursors, leading to bone marrow (BM) failure and subsequently impairing hematopoiesis (1). As acute leukemia, AML develops quickly and is typically fatal within weeks or months if left untreated (1,2). According to the Global Burden of Disease Study [2015], about one million people globally are affected by AML and 147,000 AML-induced deaths occur in 2015 (3,4). AML patients are often accompanied with various bothersome symptoms, including fatigue, shortness of breath, bone pain or tenderness, easy bruising and bleeding, fever, weight loss and so on (2). And these are associated with decreased physical and social activities, poor body image and low socioeconomic status, thereby directly and negatively impact psychosocial wellbeing in AML patients (1,5). In addition, although current treatment strategies of AML are diversified, chemotherapy is still the cornerstone of AML treatment to achieve disease remission and prolong survival. However, a wide range of side effects (e.g., nausea, vomiting, constipation, anemia, hair loss, or even secondary neoplasm) induced by chemotherapy may further deteriorate the emotional, cognitive and social functions of AML patients, and consequently lead to the occurrence of mental disorders (6-9). Previous studies have elucidated that due to the substantial symptom burden of AML and its treatment, anxiety and depression are the most frequent psychological disorders in AML patients, especially for those who were newly diagnosed (10,11). Moreover, anxiety and depression are reported to worsen the prognosis in AML patients (12-14). Thus, incorporating psychosocial interventions into usual care is necessary to help achieve better prognosis in de novo AML patients undergoing chemotherapy.
Care intervention has been considered as a common and efficient assistant strategy for rehabilitation in cancer patients undergoing chemotherapy to remedy disease/treatment-related symptoms and side effects (15-17). Emerging evidence also indicate that care intervention in varied researches with different contents and intervention intensities contributes to better outcomes in leukemia patients, such as better physical function, enhanced health-related quality of life (Qol) and higher life satisfaction (17-19). However, in previous care intervention programs for acute leukemia patients, the intervention methods are limited by short duration, low frequency, and little attention paid to the longer-term effect of care intervention on psychosocial problems and prognosis in de novo AML patients receiving chemotherapy (17-19). Thus, intensive patient’s care program (IPCP), an intensive and integrated care including AML health education, psychological guidance and telephone counselling, was devised in this study with the purpose of investigating the effect of IPCP on anxiety, depression, event-free survival (EFS) and overall survival (OS) in de novo AML patients receiving chemotherapy.
Methods
Participants
Between 2014/1/1 and 2016/6/30, 220 patients with de novo AML who underwent chemotherapy in Renmin Hospital, Hubei University of Medicine were consecutively enrolled in this study. Inclusion criteria were as follows: (I) diagnosed as primary AML according to World Health Organization (WHO) Classification of Tumors of Hematopoietic and Lymphoid Tissues [2008]; (II) age above 18 years old; (III) about to receive chemotherapy; (IV) able to complete questionnaires of assessments; (V) likely to be followed up regularly. Exclusion criteria consisted of: (I) acute promyelocytic leukemia (APL) or central nervous system leukemia (CNSL); (II) secondary AML; (III) previous exposure to radiotherapy, chemotherapy, targeted therapy, immunotherapy or allogeneic stem cell transplantation; (IV) received antidepressant or antianxiety treatment within 3 months; (V) with the comorbidity of other malignant tumors or life-threatening diseases; (VI) poorly controlled diabetes, hypertension or hyperlipidemia; (VII) severe or uncontrolled heart, renal or liver disease; (VIII) pregnancy or breastfeeding women.
Ethics
This study was approved by the Institutional Review Board of Renmin Hospital, Hubei University of Medicine and conducted in accordance with the provisions of the Declaration of Helsinki and Good Clinical Practice guidelines. All patients signed the informed consents.
Randomization procedure
In this randomized, controlled study, patients were randomly assigned to the IPCP group (n=110) or control group (n=110) as a 1:1 ratio using the block randomization method with a block size of 6. Randomization sequence was created with the use of SAS 9.3 (Statistical Analysis System, USA) statistical software by a statistical analyzer who was blind to the assignment process. The documents of randomization were sent and kept in Shanghai Qeejen Bio-tech Company (Shanghai, China). When a patient was eligible and signed the informed consent, investigator would call the Company and a unique code was provided from the randomized list.
Interventions
In the IPCP group, patients were given IPCP and usual care for 12 months, while patients in the control group were given only usual care for 12 months. The IPCP included three sections: AML health education, psychological guidance and telephone counseling, which was carried out as follows:
- AML health education: All patients were given a manual of AML health guidance in the first week post-enrollment, and the manual contained 6 parts (as shown in Table 1) including introduction of AML, prevention of bleeding, prevention of infection, healthy diet, life and mental care and discharge guidance. Then the sessions of education and instruction were conducted once a week at the Hospital (each session was 60 minutes in duration) in accordance with the manual by trained nurses in the first 3 months (M1-M3).
Table 1
Contents of AML health educationParts Subjects Contents Part 1 Introduction of AML Introduction of AML including epidemiology, pathogeny, symptom, development of treatment, prognosis and so on Part 2 Prevention of bleeding Avoid bumping injury Keep the sheets clean, avoid artificial injuries, cut nails frequently and avoid scratches Avoid scratching the skin and digging the nose emphatically, and regularly drip peppermint oil into nose to keep the mucous membrane moist Avoid a toothbrush with a hard hair Part 3 Prevention of infection To strengthen respiratory tract infection prevention, indoor items need to be cleaned, keep the air fresh, change clothes according to the temperature changes, prevent colds, and keep warm To limit the number of visitation visits, and the visitors should wear masks Gargle in the morning, before and after dinner and before sleep For skin infections, keep skin dry and clean and avoid scratches Pay attention to the prevention of perianal infection Part 4 Healthy diet Eat high protein and digestible foods and prohibit eating excellent and coarse foods Keep the defecate unobstructed Part 5 Life and mental care Keep an optimistic mood and a good state of mind Set up confidence of treatment Development of good habits Part 6 Discharge guidance Maintain adequate sleep and pay attention to personal hygiene Regular temperature measurement and outpatient review AML, acute myelocytic leukemia. - Psychological guidance: After AML health education in the first 3 months, patients received psychological guidance, which was given by the trained nurses and carried out in the way of cordial communication at the hospital or home (if discharged from hospital), every two weeks for 6 months (M4-M9). During each psychological guidance (60 minutes), patients were encouraged to communicate with the trained nurses and complain about their trouble or issues that made them anxious or distressed. Then trained nurses would comfort the patient, give emotional support, try to solve the patient’s trouble on the basis of fully understanding of the situation, meanwhile, help patient set up confidence of treatment.
- Telephone counseling: Each patient was assigned a health counselor (trained nurse) who was responsible for the telephone counseling, and counseling sessions were performed every two weeks for 3 months (M10-M12) after the accomplishment of 6-month psychological guidance. Each telephone session was conducted in 25–30 minutes, containing the following content: Firstly, the counselor would inquire current situation of the patient including body, mind, life, diet and exercise condition, then give corresponding advice to the patient for existing problems. Subsequently, according to the therapeutic status and the condition of recovery, the counselor would remind patient to review in time.
In addition, the Nursing Care Training Certification was obtained by the Wuhan Department of Health, and the nurses were trained in a training program as lecture: to practice ratio 3 to 2 in the Wuhan Department of Health.
Usual care
Both IPCP group and control group were given usual care by general nurses and primary care physicians. During hospitalization, usual care included simple healthy education of AML (which was performed only once in the first month after enrollment, introducing disease knowledge to patients about the cause, type, symptoms, treatment, prognosis and attentions of the disease), daily observation and monitoring, symptomatic supportive treatments, protective isolation, construction of a quiet environment, ultraviolet disinfection for air, strictly stipulating visiting time, instructions of medication use, diet and infection prevention and so on. During the period of discharge, usual care consisted of regular reexamination as well as regular (every 3 months) visit by telephone or outpatient.
Treatment and monitoring
All patients underwent morphology, immunology, cytogenetics and molecular biology (MICM) examination before treatment, then received Ara-C-based standard chemotherapy regimens according to disease conditions and risk stratifications. Ara-C-based induction regimens included DA, IA and MA, which were administrated as follows: (I) DA: daunorubicin at a dose of 45–90 mg/m2/d on days 1 to 3; Ara-C at a dose of 100–200 mg/m2/d on days 1 to 7; (II) IA: idarubicin at a dose of 8–12 mg/m2/d on days 1 to 3; Ara-C at a dose of 100–150 mg/m2/d on days 1 to 7; (III) MA: mitoxantrone at a dose of 8–12 mg/m2/d on days 1 to 3, Ara-C at a dose of 100–150 mg/m2/d on days 1 to 7. In the myelosuppression stage (7–14 days after completion of induction chemotherapy) and recovery stage (21–28 days after completion of induction chemotherapy), bone marrow (BM) aspirate/biopsy and blood test were performed to monitor BM condition and the blasts percentage of residual cellularity. Then treatment was adjusted according to the remission status. For patients achieved complete remission, consolidation therapies were given. If there were available donors and patients were suitable for the hematopoietic stem cell transplantation (HSCT), HSCTs were performed. As for patients without remission, they were treated as induction failures and best supportive cares were given or original regimens were repeated in accordance with the blasts percentage of residual cellularity.
Baseline characteristics collection
During the baseline period, patients’ clinical characteristics were recorded, which included age, gender, highest education, smoke, drink, hypertension, hyperlipidemia, diabetes, French-American-British (FAB) classification, cytogenetics, internal tandem duplications in the FMS-like tyrosine kinase 3 (FLT3-ITD) mutational status, isolated biallelic CCAAT/enhancer binding protein α (CEBPA) mutational status, nucleophosmin (NPM1), risk stratification as well as the level of white blood cell (WBC). The FAB classification was assessed according to FAB classification system; the cytogenetics was evaluated according to an International System for Human Cytogenetic Nomenclature (ISCN 2009); the risk status was classified according to National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology of AML (Version 2.2013).
Follow up and assessments
All patients were regularly followed up through hospitalization or telephone. During the first 12 months of follow up, Hospital Anxiety and Depression Scale (HADS) anxiety (HADS-A) score and the Zung Self-Rating Anxiety Scale (SAS) score were used to assess the anxiety of patients, as well as the HADS depression (HADS-D) score and the Zung Self-Rating Depression Scale (SDS) score were used to assess the depression of patients. Both the HADS and the SAS/SDS were assessed at baseline (M0), month 3 (M3), M6, M9 and M12. As for patients who did not complete 12-month follow up, intention-to-treat (ITT) analysis was applied, and the scores at the last follow up were used as the values of each later missing visit. After that, patients were further followed up until 2017/6/30, and the median follow-up duration was 20.0 months (range, 2.0–36.0 months). Finally, all 220 patients were included in the survival analysis, and the data of patients who lost follow up were treated as censored data. Besides, EFS was calculated from the date of entry into the study to the date of recurrence, progression or death from any cause, and OS was calculated from the date of entry into the study to the date of death from any cause.
Definitions
The HADS-A/HADS-D and SAS/SDS were frequently used self-rating scales developed to measure symptoms of anxiety and depression. Both HADS-A and HADS-D included 7 questions, and each question was scored as 0–3 points individually, resulting in 0–21 points and being classified as: 0–7, no anxiety/depression; 8–10, light anxiety/depression; 11–14, moderate anxiety/depression; 15–21, severe anxiety/depression (20); Both SAS and SDS contained 20 questions, and each question was scored as 1–4 points individually, ranging from “none or a little of the time” to “most or all of the time”, resulting in 20–80 raw score, subsequently standard scores were calculated by int (1.25*raw score) and classified as: 25–49, no anxiety/depression; 50–59, light anxiety/depression; 60-69, moderate anxiety/depression; 70–100, severe anxiety/depression (21,22). In addition, sustained anxiety patient was defined as the patient with anxiety at M0 and M12 evaluated by the HADS-A or SAS score. Similarly, sustained depression patient was defined as the patient with depression at M0 and M12 assessed by the HADS-D or SDS score.
Statistics analysis
Statistical analysis was performed with the use of the SPSS 22.0 software (IBM, USA). Randomization was carried out using the SAS 9.3 (Statistical Analysis System, USA) statistical software and figures were made by Graphpad Prism 6.01 software (GraphPad Software Inc., USA). Data were presented as mean value ± standard deviation, median value (1/4–3/4 quantiles) or count (percentage). Comparison between two groups was determined by t-test, Wilcoxon rank sum test or Chi-square test. Kaplan-Meier (K-M) curves and log-rank test were used to assess the difference of EFS and OS between two groups. P value <0.05 was considered statistically significant.
Results
Study flow
This study consisted of two stages: randomized, controlled stage and long-term follow up stage (Figure 1). In the randomized, controlled stage, a total of 436 AML patients were invited into this study, while 81 patients were excluded, including 64 cases who missed invitation and 17 cases who declined to attend the pre-screening procedure. Subsequently, there were 355 patients screened for eligibility, and 135 cases were excluded including 53 exclusions and 82 cases disagreed to sign informed consents. The remaining 220 patients were then randomized as 1:1 radio, among whom, 110 cases were allocated to IPCP group receiving IPCP and usual care for 12 months, while the other 110 cases were allocated to control group only receiving usual care for 12 months. In IPCP group, 18 cases withdrew during the study including 4 cases who lost follow up and 14 deaths, leaving 92 cases who completed the 12-month follow up. In control group, there were total 24 withdrawals, which included 2 cases who lost follow up and 22 deaths, leading to 86 cases who completed the 12-month follow up.
In the long-term follow up stage, patients were further followed up until 2017/6/30, and the median follow-up duration was 20.0 months (range, 2.0–36.0 months). During this stage, there were 10 cases who lost follow up and 25 deaths in IPCP group, while in control group, there were 11 cases who lost follow up and 26 deaths. All 220 patients in IPCP and control groups were included in survival analysis (The data of patients who lost follow up were treated as censored data).
Baseline characteristics of AML patients
There was no difference in baseline characteristics between IPCP group (n=110) and control group (n=110) (All P>0.05, Table 2). In brief, mean age was 43.7±13.9 years and 44.0±14.8 years in IPCP group and control group, respectively (P=0.873). The numbers of male and female were 52 and 58 in IPCP group, 60 and 50 in control group (P=0.281). There were 28 (25.5%) patients in IPCP group and 34 (30.9%) patients in control group who were with smoke history (P=0.369). The numbers of patients with hypertension (P=0.357), hyperlipidemia (P=0.676) and diabetes (P=0.757) were 15 (13.6%), 12 (10.9%) and 5 (4.5%) respectively in IPCP group, 20 (18.2%), 14 (12.7%) and 6 (5.5%) respectively in control group. The numbers of patients with FAB classification of M1, M2, M3, M4, M5 and M6 were 0 (0.0%), 52 (47.3%), 26 (23.6%), 29 (26.4%) and 3 (2.7%) respectively in IPCP group, and 2 (1.8%), 36 (32.7%), 33 (30.0%), 32 (29.1%) and 7 (6.4%) respectively in control group (P=0.112). In addition, according to risk stratification, the numbers of patients with better-risk, intermediate-risk and poor-risk were 45 (40.9%), 44 (40.0%) and 21 (19.1%) respectively in IPCP group, 41 (37.3%), 40 (36.4%) and 29 (26.4%) respectively in control group (P=0.437). Other detailed baseline characteristics were shown in Table 2.
Table 2
| Items | IPCP group (N=110) (n/%) | Control group (N=110) (n/%) | P value |
|---|---|---|---|
| Age (years) | 43.7±13.9 | 44.0±14.8 | 0.873 |
| Gender (male/female) | 52/58 | 60/50 | 0.281 |
| Highest education | 0.872 | ||
| Primary school or less | 20 (18.2) | 21 (19.1) | |
| High school | 48 (43.6) | 42 (38.2) | |
| Undergraduate | 34 (30.9) | 38 (34.5) | |
| Graduate or above | 8 (7.3) | 9 (8.2) | |
| Smoke | 28 (25.5) | 34 (30.9) | 0.369 |
| Drink | 34 (30.9) | 36 (32.7) | 0.772 |
| Hypertension | 15 (13.6) | 20 (18.2) | 0.357 |
| Hyperlipidemia | 12 (10.9) | 14 (12.7) | 0.676 |
| Diabetes | 5 (4.5) | 6 (5.5) | 0.757 |
| FAB classification | 0.112 | ||
| M1 | 0 (0.0) | 2 (1.8) | |
| M2 | 52 (47.3) | 36 (32.7) | |
| M4 | 26 (23.6) | 33 (30.0) | |
| M5 | 29 (26.4) | 32 (29.1) | |
| M6 | 3 (2.7) | 7 (6.4) | |
| Cytogenetics | 0.279 | ||
| t(6;9) | 1 (0.9) | 0 (0.0) | |
| t(9;22) | 1 (0.9) | 0 (0.0) | |
| inv(3) or t(3;3) | 2 (1.8) | 0 (0.0) | |
| t(9;11) | 2 (1.8) | 0 (0.0) | |
| 11q23 | 0 (0.0) | 3 (2.7) | |
| -5 or 5q- | 2 (1.8) | 2 (1.8) | |
| -7 or 7q- | 1 (0.9) | 3 (2.7) | |
| +8 | 4 (3.6) | 2 (1.8) | |
| CK | 3 (2.7) | 9 (8.2) | |
| inv(16) or t(16;16) | 11 (10.0) | 8 (7.3) | |
| t(8;21) | 11 (10.0) | 10 (9.1) | |
| Others (not included in better or poor risk) | 12 (10.9) | 14 (12.7) | |
| NK | 60 (54.5) | 59 (53.6) | |
| Monosomal karyotype | 5 (4.5) | 8 (7.3) | 0.391 |
| FLT3-ITD mutation | 20 (18.2) | 21 (19.1) | 0.863 |
| Isolated biallelic CEBPA mutation | 10 (9.1) | 9 (8.2) | 0.810 |
| NPM1 | 40 (36.4) | 40 (36.4) | 1.000 |
| Risk stratification | 0.437 | ||
| Better-risk | 45 (40.9) | 41 (37.3) | |
| Intermediate-risk | 44 (40.0) | 40 (36.4) | |
| Poor-risk | 21 (19.1) | 29 (26.4) | |
| WBC (×109 cell/L) | 11.32 (4.94–22.39) | 11.82 (5.89–20.40) | 0.817 |
Data were presented as mean value ± standard deviation, median value (1/4–3/4 quartiles) or count (percentage). Comparison was determined by t-test, Wilcoxon rank sum test or Chi-square test. P value <0.05 was considered statistically significant. AML, acute myeloid leukemia; IPCP, intensive patients’ care program; FAB Classification, French-American-British classification system; CK, complex karyotype; NK, normal karyotype; FLT3-ITD, internal tandem duplications in the FMS-like tyrosine kinase 3; CEBPA, CCAAT/enhancer binding protein α; NPM1, nucleophosmin; WBC, white blood cell.
Baseline assessments of anxiety and depression level in AML patients
The baseline anxiety and depression level in IPCP group and control group were listed in Table 3, which disclosed that no difference concerning the HADS-A score (P=0.737), anxiety severity by HADS-A score (P=0.293), SAS score (P=0.367), anxiety severity by SAS score (P=0.425), HADS-D score (P=0.806), depression severity by HADS-D score (P=0.405), SDS score (P=0.853) or depression severity by SDS score (P=0.902) was observed between IPCP and control groups.
Table 3
| Items | IPCP group (N=110) (n/%) | Control group (N=110) (n/%) | P value |
|---|---|---|---|
| HADS-A score | 6.2±3.1 | 6.3±3.7 | 0.737a |
| Anxiety severity by HADS-A score | 0.293b | ||
| No anxiety | 80 (72.7) | 73 (66.4) | |
| Light | 20 (18.2) | 24 (21.8) | |
| Moderate | 8 (7.3) | 9 (8.2) | |
| Severe | 2 (1.8) | 4 (3.6) | |
| SAS score | 44.4±10.4 | 45.7±10.3 | 0.367a |
| Anxiety severity by SAS score | 0.425b | ||
| No anxiety | 82 (74.5) | 76 (69.1) | |
| Light | 15 (13.6) | 21 (19.1) | |
| Moderate | 12 (10.9) | 11 (10.0) | |
| Severe | 1 (0.9) | 2 (1.8) | |
| HADS-D score | 6.2±2.7 | 6.1±3.7 | 0.806a |
| Depression severity by HADS-D score | 0.405b | ||
| No depression | 85 (77.3) | 82 (74.5) | |
| Light | 19 (17.3) | 11 (10.0) | |
| Moderate | 5 (4.5) | 14 (12.7) | |
| Severe | 1 (0.9) | 3 (2.7) | |
| SDS score | 43.7±10.3 | 43.5±10.7 | 0.853a |
| Depression severity by SDS score | 0.902b | ||
| No depression | 82 (74.5) | 83 (75.5) | |
| Light | 17 (15.5) | 16 (14.5) | |
| Moderate | 10 (9.1) | 9 (8.2) | |
| Severe | 1 (0.9) | 2 (1.8) |
Data were presented as mean value ± standard deviation or count (percentage). a, Comparison was determined by t-test; b, comparison was determined by Wilcoxon rank sum test. P value <0.05 was considered statistically significant. Both HADS-A and HADS-D were classified as: 0–7, no anxiety/depression; 8–10, light anxiety/depression; 11–14, moderate anxiety/depression; 15–21, severe anxiety/depression; both SAS and SDS were classified as: 25–49, no anxiety/depression; 50–59, light anxiety/depression; 60–69, moderate anxiety/depression; 70–100, severe anxiety/depression. AML, acute myeloid leukemia; IPCP, intensive patient’s care program; HADS-A score, Hospital Anxiety and Depression Scale anxiety score; HADS-D score, Hospital Anxiety and Depression Scale depression score; SAS score, Zung Self-Rating Anxiety Scale; SDS score, Zung Self-Rating Depression Scale.
Comparison of anxiety assessed by HADS-A or SAS score between IPCP group and control group
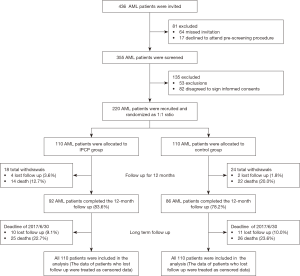
HADS-A score was lower at M12 in IPCP group compared to control group (P<0.05), while no difference was found between two groups at M0, M3, M6 or M9 (All P>0.05, Figure 2A). As to HADS-A score change (M12-M0), its decrease was larger in IPCP group (−1.14±2.50) compared to control group (−0.37±3.20) (P=0.050, Figure 2B). Meanwhile, the anxiety rate (%) by HADS-A score at M12 was 16.4% in IPCP group, which was lower than that in control group (29.1%) (P=0.024, Figure 2C). In addition, the anxiety severity by HADS-A score at M12 was lower in IPCP group compared with control group (P=0.020, Figure 2D).
With respect to SAS score, it was lower in IPCP group compared to control group at M6 (P<0.05), M9 (P<0.01) and M12 (P<0.001), while no difference was found at M0 or M3 between two groups (All P>0.05, Figure 2E). The SAS score change (M12-M0) seemed to be greater in IPCP group (−4.71±10.68) compared to control group (−1.73±12.16), but without statistical significance (P=0.055, Figure 2F). Additionally, no difference was found in anxiety rate (%) by HADS-A score at M12 (P=0.178, Figure 2G) or anxiety severity by HADS-A score at M12 (P=0.156, Figure 2H) between IPCP and control groups.
Comparison of depression assessed by HADS-D or SDS score between IPCP group and control group
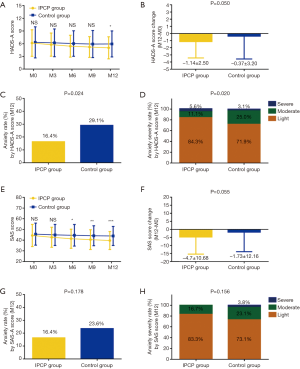
HADS-D score was lower at M12 in IPCP group compared to control group (P<0.05), while no difference was found at M0, M3, M6 or M9 between the two groups (All P>0.05, Figure 3A). Regarding HADS-D score change (M12-M0), its reduction was greater in IPCP group (−1.46±2.93) than that in control group (−0.45±4.28) (P=0.043, Figure 3B). However, there was no difference in depression rate (%) by HADS-D score at M12 (P=0.145, Figure 3C) or depression severity by HADS-D score at M12 (P=0.150, Figure 3D) between IPCP and control groups.
Similarly, SDS score at M12 was lower in IPCP group compared to control group (P<0.05), while no difference was found at M0, M3, M6 or M9 between two groups (All P>0.05, Figure 3E). Furthermore, the SDS score change (M12-M0) was larger in IPCP group (−4.50±9.62) compared to control group (−1.32±12.18) (P=0.033, Figure 3F). However, no difference was detected in depression rate (%) by HADS-D score at M12 (P=0.162, Figure 3G) or depression severity by HADS-D score at M12 (P=0.235, Figure 3H) between IPCP and control groups.
Comparison of EFS and OS between IPCP group and control group
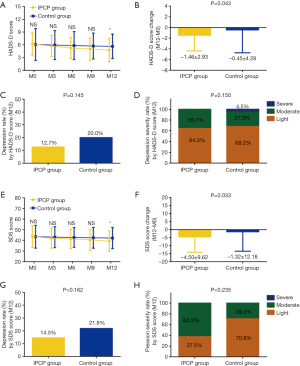
In the long-term follow up stage, K-M curve and log-rank test were used to evaluate the difference of EFS and OS between IPCP and control groups, which revealed that compared to control group (17.5 months, 95% CI: 15.3–19.7 months), IPCP group (20.8 months, 95% CI: 18.5–23.1 months) showed an increased trend of EFS, but no statistical significance was observed (P=0.071, Figure 4A). As to OS, IPCP group (28.2 months, 95% CI: 26.3–30.2 months) illustrated a better OS compared to control group (24.4 months, 95% CI: 22.2–26.6 months) (P=0.045, Figure 4B).
Influence of sustained anxiety or sustained depression on EFS or OS in AML patients underwent chemotherapy
To investigating the influence of sustained anxiety by HADS-D/SDS score on EFS, the comparison between patients with sustained anxiety and others were performed, which illustrated that no difference of EFS was detected between patients with sustained anxiety by HADS-D (P=0.514, Figure 5A)/SDS score (P=0.765, Figure 5B) and others. Similarly, as to the influence of sustained depression on EFS, there was no difference of EFS between patients with sustained depression by HADS-D score (P=0.483, Figure 5C) or SDS score (P=0.569, Figure 5D) and others.
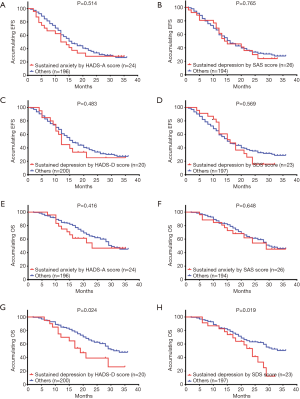
Regarding the impact of sustained anxiety on OS, no difference of OS between patients with sustained anxiety by HADS-A score (P=0.416, Figure 5E) or SAS score (P=0.648, Figure 5F) and others was found. However, patients with sustained depression by HADS-D score exhibited a worse OS compared to others (P=0.024, Figure 5G). Meanwhile, OS in patients with sustained depression by SDS score was also shorter compared to others (P=0.019, Figure 5H).
Discussion
In this present study, we observed that: (I) IPCP decreased anxiety and depression assessed by HADS-A/D or SAS/SDS score in de novo AML patients who underwent chemotherapy. (II) OS was prolonged in IPCP group compared to control group, and patients with sustained depression assessed by HADS-D or SDS score disclosed worse OS compared to non-sustained depression patients.
AML is an aggressive hematologic disease characterized by abrupt onset, intensive therapeutic schedule, relatively poor prognosis, and high risk of relapse (1,23). De novo AML patients face substantial challenges regarding physical deconditioning, impaired mobility and quality of life (Qol), which have enormous negative influence on psychological health (12-14). Previous care intervention (e.g., psychological guidance, counseling, exercise and so on) has been illustrated to play positive roles in patients’ adapting to leukemia and dealing with its consequent psychological problems, such as anxiety and depression (10,11,16,17,19). For example, two randomized clinical trials jointly reveal that emotional support implemented for 3 days decreases anxiety and depression levels in leukemia patients by encouraging them to express their feelings, needs and concerns, and then giving corresponding help (10,11). Additionally, another randomized controlled trial illustrates that three-week walking exercise program alleviates anxiety and depression in AML patients during chemotherapy (16). Nevertheless, a self-control study in acute leukemia patients undergoing intensive chemotherapy displays that the six-week health counseling and exercise intervention could reduce physical sufferings and improve Qol. However, anxiety and depression only show a decreased trend but without statistical significance (17). In view of the obscure and uncertain results about the effect of care intervention on anxiety and depression in previous studies, we designed IPCP, an intensive and integrated program with three core contents, including AML health education, psychological guidance and telephone counselling, to validate its positive role in psychological management for AML patients, and the results verified that IPCP alleviated anxiety and depression symptoms in de novo AML patients underwent chemotherapy. These might be explained through the three core contents of IPCP: (I) the weekly AML health education in the first three months provided patients with a detailed description of the disease, its therapeutic interventions, possible side effects and their prevention measures, prognosis, guidance on diet and mentality, which contributed to the reduction of panic, fear and uncertainty, as well as the increase of patients’ confidence during the early phase of chemotherapy, thereby decreasing patients’ anxiety and depression levels. In addition, the AML health education in this program was quite different from the usual education in clinical practice, which was due to that content of the manual was more comprehensive, the nurses were trained specifically for the content of the manual and the education was carried out more regularly. (II) Psychological guidance conducted by the trained nurses could help patients to vent negative emotions and talk about unpleasant experiences that induced anxiety or distress during chemotherapy. Then the trained nurses provided emotional support to fulfill patients’ spiritual needs and help them get out of the negative mood states. (III) Telephone counselling could track the physiology and psychology conditions of AML patients even though they were discharged, then, health counselors instructed patients to self-monitor at home, gave suggestions for reducing the risk of complications, encouraged low or moderate intensity exercise and reminded them of out-patient review regularly, which might help AML patients to maintain good physical and mental health, thereby reducing the risk of anxiety and depression in AML patients.
Recent studies also indicate the favorable effects of care interventions on prognosis in leukemia patients (18,19). For instance, a prospective study discloses that the survival time in leukemia patients with emotional support is longer compared to those without emotional support, suggesting that emotional support may have positive effect on survival (18). Furthermore, anxiety and depression have been shown to be inseparable with poor prognosis in various cancers, including leukemia, and patients with longer survival often present with lower levels of anxiety and depression (24-26). Based on aforementioned evidence, we hypothesized that care intervention may have positive effect on survival of AML patients probably through ameliorating anxiety and depression, while few studies have been conducted to investigate the correlation of anxiety and depression with survival interval during the period of care intervention in AML patients receiving chemotherapy. In order to verify our hypothesis, the DFS and OS were compared between patient with sustained anxiety/depression and patients without anxiety/depression, which disclosed that OS was longer in IPCP group compared to control group and patients with sustained depression assessed by HADS-D or SDS score presented with prolonged OS compared to others. And these suggested that IPCP might prolong OS through alleviating sustained depression in de novo AML patients undergoing chemotherapy. Moreover, it is reported that AML patients who receive intensive or non-intensive chemotherapy present with less possibility of cure (27).
Although some interesting results were observed in this study, several limitations still existed as follows: (I) the follow-up duration was relatively short with a median value of 20.0 months, thus longer-term effect of IPCP on prognosis of de novo AML patients undergoing chemotherapy was not investigated. (II) The sample size in the current study was relatively small which might result in low statistical power and less significant clinical difference. (III) All patients in this study were recruited from single tertiary hospital, thus, the generalizability may be limited. (IV) As a randomized, controlled study, the clinicians were not blinded to the interventions, which might cause selection bias.
Conclusions
In conclusion, IPCP decreases anxiety and depression as well as improves OS in de novo AML patients underwent chemotherapy, which highlights a new dimension in care intervention to obtain better prognosis in AML patients.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.01.32). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Renmin Hospital, Hubei University of Medicine and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Döhner H, Weisdorf DJ, Bloomfield CD. Acute Myeloid Leukemia. N Engl J Med 2015;373:1136-52. [Crossref] [PubMed]
- Estey E. Acute Myeloid Leukemia - Many Diseases, Many Treatments. N Engl J Med 2016;375:2094-5. [Crossref] [PubMed]
- GBD 2015 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1545-602. [Crossref] [PubMed]
- GBD 2015 Mortality and Causes of Death Collaborators . Causes of Death C. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1459-544. [Crossref] [PubMed]
- Bryant AL, Deal AM, Battaglini CL, et al. The Effects of Exercise on Patient-Reported Outcomes and Performance-Based Physical Function in Adults With Acute Leukemia Undergoing Induction Therapy: Exercise and Quality of Life in Acute Leukemia (EQUAL). Integr Cancer Ther 2018;17:263-70. [Crossref] [PubMed]
- Ebrahimi M, Allahyari A, Ebrahimi M, et al. Effects of Dietary Honey andArdehCombination on Chemotherapy- Induced Gastrointestinal and Infectious Complications in Patients with Acute Myeloid Leukemia: A Double-Blind Randomized Clinical Trial. Iran J Pharm Res 2016;15:661-8. [PubMed]
- Altman JK, Foran JM, Pratz KW, et al. Phase 1 study of quizartinib in combination with induction and consolidation chemotherapy in patients with newly diagnosed acute myeloid leukemia. Am J Hematol 2018;93:213-21. [Crossref] [PubMed]
- Rüther U, Nunnensiek C, Schmoll HJ. Secondary Neoplasias following Chemotherapy, Radiotherapy, and Immunosuppression. Karger 2004. doi:
10.1159/isbn.978-3-318-00615-5 . - Cheng MJ, Smith BD, Hourigan CS, et al. A Single Center Survey of Health-Related Quality of Life among Acute Myeloid Leukemia Survivors in First Complete Remission. J Palliat Med 2017;20:1267-73. [Crossref] [PubMed]
- Moeini M, Taleghani F, Mehrabi T, et al. Effect of a spiritual care program on levels of anxiety in patients with leukemia. Iran J Nurs Midwifery Res 2014;19:88-93. [PubMed]
- Musarezaie A, Moeini M, Taleghani F, et al. Does spiritual care program affect levels of depression in patients with Leukemia? A randomized clinical trial. J Educ Health Promot 2014;3:96. [PubMed]
- Amler S, Sauerland MC, Deiters C, et al. Factors influencing life satisfaction in acute myeloid leukemia survivors following allogeneic stem cell transplantation: a cross-sectional study. Health Qual Life Outcomes 2015;13:28. [Crossref] [PubMed]
- El-Jawahri A, Chen YB, Brazauskas R, et al. Impact of pre-transplant depression on outcomes of allogeneic and autologous hematopoietic stem cell transplantation. Cancer 2017;123:1828-38. [Crossref] [PubMed]
- Albrecht TA, Boyiadzis M, Elswick RK Jr, et al. Symptom Management and Psychosocial Needs of Adults With Acute Myeloid Leukemia During Induction Treatment: A Pilot Study. Cancer Nurs 2017;40:E31-8. [Crossref] [PubMed]
- Salins N, Ramanjulu R, Patra L, et al. Integration of Early Specialist Palliative Care in Cancer Care and Patient Related Outcomes: A Critical Review of Evidence. Indian J Palliat Care 2016;22:252-7. [Crossref] [PubMed]
- Chang PH, Lai YH, Shun SC, et al. Effects of a walking intervention on fatigue-related experiences of hospitalized acute myelogenous leukemia patients undergoing chemotherapy: a randomized controlled trial. J Pain Symptom Manage 2008;35:524-34. [Crossref] [PubMed]
- Jarden M, Adamsen L, Kjeldsen L, et al. The emerging role of exercise and health counseling in patients with acute leukemia undergoing chemotherapy during outpatient management. Leuk Res 2013;37:155-61. [Crossref] [PubMed]
- Grulke N, Bailer H, Hertenstein B, et al. Coping and survival in patients with leukemia undergoing allogeneic bone marrow transplantation--long-term follow-up of a prospective study. J Psychosom Res 2005;59:337-46. [Crossref] [PubMed]
- Leak Bryant A, Walton AL, Pergolotti M, et al. Perceived Benefits and Barriers to Exercise for Recently Treated Adults With Acute Leukemia. Oncol Nurs Forum 2017;44:413-20. [Crossref] [PubMed]
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361-70. [Crossref] [PubMed]
- Gainotti G, Cianchetti C, Taramelli M, et al. The guided self-rating anxiety-depression scale for use in clinical psychopharmacology. Act Nerv Super (Praha) 1972;14:49-51. [PubMed]
- Zung WW, Gianturco JA. Personality dimension and the Self-Rating Depression Scale. J Clin Psychol 1971;27:247-8. [Crossref] [PubMed]
- Ghodraty-Jabloo V, Alibhai SMH, Breunis H, et al. Keep your mind off negative things: coping with long-term effects of acute myeloid leukemia (AML). Support Care Cancer 2016;24:2035-45. [Crossref] [PubMed]
- Liu J, Zong G, Zhang C, et al. Anxiety and serum catecholamines as predictors of survival and recurrence in hepatocellular carcinoma. Psychooncology 2017;26:1347-53. [Crossref] [PubMed]
- Vodermaier A, Lucas S, Linden W, et al. Anxiety After Diagnosis Predicts Lung Cancer-Specific and Overall Survival in Patients With Stage III Non-Small Cell Lung Cancer: A Population-Based Cohort Study. J Pain Symptom Manage 2017;53:1057-65. [Crossref] [PubMed]
- Akaho R, Sasaki T, Mori S, et al. Psychological factors and survival after bone marrow transplantation in patients with leukemia. Psychiatry Clin Neurosci 2003;57:91-6. [Crossref] [PubMed]
- El-Jawahri A, Nelson-Lowe M, VanDusen H, et al. Patient-Clinician Discordance in Perceptions of Treatment Risks and Benefits in Older Patients with Acute Myeloid Leukemia. Oncologist 2019;24:247-54. [Crossref] [PubMed]