The high expression of TNF-α and NF-κB in tumor microenvironment predicts good prognosis of patients with BCLC-0-B hepatocellular carcinoma
Introduction
The main pathological type of primary liver cancer is hepatocellular carcinoma (HCC) (1). As one of the most common malignancies in the world, liver cancer has become the fourth leading cause of cancer death in the world, and it is the second leading cause of male cancer death in the world (2). In China, liver cancer has become the third most common tumour and the fourth leading cause of cancer death in men and women (3). In recent years, the incidence and mortality of liver cancer in China have been rising, seriously threatening the health and lives of our people (3). Although in recent years, surgical resection, ablation treatment, liver transplantation and other methods have made some progress in the treatment of HCC, the prognosis of HCC patients is still poor due to its high recurrence rate and mortality. Therefore, studying factors that influence survival and recurrence in patients with HCC is important to improve patient survival.
In recent years, an increasing number of studies have shown that inflammatory factors in the HCC microenvironment play an important role in the development of HCC. Many inflammatory factors are present in the microenvironment of HCC, such as tumor necrosis factor-alpha (TNF-α), IL-1, and IL-6. The interaction between hepatocytes and inflammatory factors leads to changes in the microenvironment, which are crucial in the early and late stages of HCC. Among these factors, TNF-α is mainly produced by activated monocyte-macrophages, endothelial cells, and lymphocytes, and it exhibits biological activity by binding to soluble TNF-binding receptors (TNFR1 and TNFR2) (4). TNF-α binds to its cell membrane receptor and activates intracellular downstream signalling pathways, including proapoptotic pathways induced by caspase-8 interaction with Fas-associated death domain (FADD) and the cIAP-1 and TRAF2 interaction-induced resistance apoptotic pathway (5-8).
However, many controversies remain in the research reports on TNF-α. TNF-α often exhibits opposite biological functions during tumorigenesis and development (9,10). TNF-α was initially discovered to be the most effective anticancer cytokine. Local, high-dose TNF-α treatment of tumours can selectively destroy tumour tissue blood vessels, thereby exerting anti-tumour effects (11). In the process of tumour treatment, serum TNF-α was found to be negatively correlated with tumour area and tumour number (12). In addition, other studies have shown that diclofenac can cause mild apoptosis in HepG2 cells, but this effect can be greatly enhanced by TNF-α (13). Interestingly, however, studies have shown that in the early stages of HCC, loss of TNFR1 signalling in mice reduces the development of liver cancer (14). In the late stage of HCC development, anti-TNF-α treatment can inhibit the progression of HCC (15). These studies indicate that TNF-α is involved in the development of HCC. Overall, the mechanism of action of TNF-α in the progression of HCC is unclear but appears to be very important. These previous studies also suggest that it is necessary to further examine the effects of TNF-α on the survival and prognosis of patients with HCC. Nuclear factor-kappa B (NF-κB) dissociates from IKB after receiving the appropriate stimuli (such as TNF-α) and enters the nucleus to affect the expression of apoptosis-related genes (8,16), thus mediating the invasion and spread of tumour cells. To further investigate the relationship between TNF-α and NF-κB levels in the HCC microenvironment and postoperative recurrence and survival in patients with HCC, we used immunohistochemical methods to identify HCC tissues from 81 patients with HCC who met the inclusion criteria. The levels of TNF-α and NF-κB were detected, and the intrinsic relationship between these factors and clinicopathological features was analysed. The risk factors for recurrence and survival after HCC operation were explored, and the results may provide a basis for estimation of postoperative recurrence in patients with HCC.
Methods
Background information
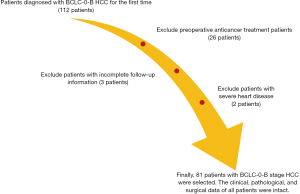
A total of 81 paraffin-embedded specimens from Barcelona Clinic Liver Cancer-0-B (BCLC-0-B) HCC patients who underwent surgical resection in our hospital from January 2000 to December 2012 were collected. There were 65 males and 16 females, with an age range of 31–83 years and a median age of 56 years. The detailed inclusion criteria are as follows: (I) patients diagnosed with BCLC-0-B HCC for the first time; (II) no anticancer treatment and radical hepatectomy before surgery; (III) follow-up information available; (IV) no autoimmune diseases, other malignant tumours or severe heart, lung, kidney or blood diseases; (V) clinical, pathological, surgical and other related data intact. Patients with non-tumour related deaths were excluded (see Figure 1 for details). All wax block sections were reviewed by two independent, experienced pathologists. This study and related use of the patients’ samples were approved by the Ethics Committee of The Affiliated Hospital of Qingdao University, and all the participants gave informed consent before taking part.
Immunohistochemistry and scoring standards
All 81 wax specimens were serially sectioned to a thickness of 4 µm. TNF-α and NF-κB were measured using immunohistochemistry, and rabbit anti-human NF-κB and TNF-α antibodies were purchased from Abcam, USA. Two pathologists, who were blinded to the clinical data, interpreted the results simultaneously. If the interpretations were different, the results were discussed with a third party until a consensus was reached. Under an optical microscope, positively stained cells displayed yellow or brown coloring in the cytoplasm. A total of ten fields were observed under high magnification (×400), and the percentage of positive cells was calculated. Cells without coloring scored 0, while those displaying light yellow, brown and tan scored 1, 2 and 3 points, respectively. A second score was obtained by determining the percentage of positive cells: <5%, ≥5%, ≥26%, ≥51% and ≥75% scored 0, 1, 2, 3 and 4 points, respectively. The two scores were summed, and a total of ≤4 represented low expression levels, while a score of >4 represented high expression levels. All patients were divided into high or low groups according to the results from the IHC. Patients with high expression levels of TNF-α and NF-κB were classified as the high expression group, while those with low expression levels of TNF-α and NF-κB, high TNF-α and low NF-κB, or low TNF-α and high NF-κB expression levels were designated as the low expression group. Additional, serum aspartate aminotransferase (AST) level are lower than or equal to 42 U/L was considered low group, serum AST level are higher than 42 U/L was considered high group.
Follow-up
All 81 patients with HCC were followed up by outpatient visit, telephone or letter to observe postoperative recurrence. The follow-up plan was every 3 months until 2 years after surgery, every 6 months between 2 and 5 years, and review every 1 year after 5 years. The review included liver function, AFP, abdominal ultrasound, and chest radiograph; if necessary, enhanced CT or MRI, needle biopsy and other tests were performed. Recurrence was defined as new lesions in the liver or outside the liver as confirmed by imaging studies or biopsy. Disease-free survival (DFS) time was defined as the time from the date of surgery to the time of recurrence or follow-up. Overall survival (OS) time is defined as the time from the date of surgery to the end of follow-up or death. Both DFS and OS were calculated on a monthly basis. The deadline for follow-up was March 2017.
Statistical analysis
Statistical analysis was performed using SPSS 24.0 software. Pearson’s Chi-square test, continuous correction Chi-square test or Fisher’s exact test was used to compare clinical pathological categorical variables. Survival curves were described using the Kaplan-Meier method and DFS and OS were compared using a log-rank test. Cox regression analysis and Cox proportional hazard models were used to calculate prognostic values for different variables. All statistical tests were bilateral and significant P values <0.05.
Results
Clinical pathological features and expression of TNF-α and NF-κB In HCC tissues, TNF-α is mainly found in the cytoplasm and nucleus of tumour cells, and NF-κB is mainly expressed in the cell membrane and cytoplasm of tumour cells (Figure 2). The relationship between the expression of TNF-α and NF-κB and clinicopathological variables was analysed by χ2 test. The results showed that the expression of TNF-α and NF-κB in HCC patients was not related to clinicopathological variables (Table 1).
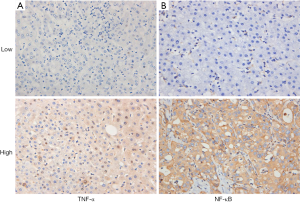
Table 1
| Parameters | All cases | TNF-α and NF-κB | χ2 | P | |
|---|---|---|---|---|---|
| Low | High | ||||
| Gender | 0.03 | 0.863a | |||
| Male | 65 | 35 | 30 | ||
| Female | 16 | 9 | 7 | ||
| Age (y) | 0.345 | 0.557a | |||
| <50 | 20 | 12 | 8 | ||
| ≥50 | 61 | 32 | 29 | ||
| Alcohol abuse | 0.001 | 0.980a | |||
| Yes | 22 | 12 | 10 | ||
| No | 59 | 32 | 27 | ||
| Tumor size (cm) | 0.174 | 0.676a | |||
| ≤5 | 63 | 35 | 28 | ||
| >5 | 18 | 9 | 9 | ||
| Tumor margin (cm) | 0.000 | 1.000 b | |||
| ≤2 | 72 | 39 | 33 | ||
| >2 | 9 | 5 | 4 | ||
| Pathologic differentiation | 0.029 | 0.866a | |||
| Middle and low | 62 | 34 | 28 | ||
| High | 19 | 10 | 9 | ||
| Microvascular tumor thrombus | 0.435 | 0.510a | |||
| No | 66 | 37 | 29 | ||
| Yes | 15 | 7 | 8 | ||
| Liver cirrhosis | 0.076 | 0.783b | |||
| No | 72 | 40 | 32 | ||
| Yes | 9 | 4 | 5 | ||
| Child-Pugh grade | — | 0.457c | |||
| A | 80 | 44 | 36 | ||
| B | 1 | 0 | 1 | ||
| Capsule invasion | 1.117 | 0.291b | |||
| No | 6 | 5 | 1 | ||
| Yes | 75 | 39 | 36 | ||
| AFP level (ng/L) | 0.345 | 0.557a | |||
| ≤400 | 61 | 32 | 29 | ||
| >400 | 20 | 12 | 8 | ||
| ALB level (g/L) | 0.972 | 0.324b | |||
| ≤35 | 9 | 3 | 6 | ||
| >35 | 72 | 41 | 31 | ||
| ALT level (U/L) | 1.522 | 0.217a | |||
| ≤60 | 66 | 38 | 28 | ||
| >60 | 15 | 6 | 9 | ||
| AST level (U/L) | 0.930 | 0.335a | |||
| ≤42 | 61 | 35 | 26 | ||
| >42 | 20 | 9 | 11 | ||
| PLT level (104/μL) | 0.128 | 0.721a | |||
| ≤10 | 19 | 11 | 8 | ||
| >10 | 62 | 33 | 29 | ||
| TBIL level (μmol/L) | 1.654 | 0.198a | |||
| ≤22 | 70 | 40 | 30 | ||
| >22 | 11 | 4 | 7 | ||
a, Pearson’s χ2 test; b, continuity correction by χ2 test; c, Fisher’s exact test; TBIL, total bilirubin; ALB, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; PLT, platelet; AFP, α-fetoprotein; TNF-α, tumor necrosis factor-alpha; NF-κB, nuclear factor-kappa B.
Survival analysis
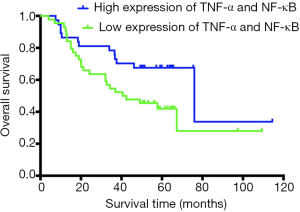
Survival analysis was performed in 81 patients with HCC using Kaplan-Meier analysis. The median survival time of the TNF-α/NF-κB high-coexpression group was 76 months (95% CI, 34.167–117.833), and the median survival time of the low-expression group was 40.6 months (95% CI, 15.645–65.555). Kaplan-Meier analysis of the relationship between the expression of TNF-α and NF-κB and OS after surgery showed that the median OS (76 vs. 40.6 months, P=0.042, Figure 3) in the high expression of TNF-α/NF-κB group were significantly longer than low expression of TNF-α/NF-κB group.
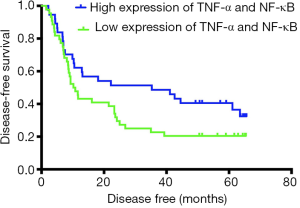
The median DFS time was 35.3 months (95% CI, 0.142–70.458) in the high-expression group and 10.2 months (95% CI, 7.275–13.125) in the low-expression group. Kaplan-Meier analysis was used to compare the postoperative DFS of the TNF-α/NF-κB high-coexpression group and the low-expression group. The median DFS (35.3 vs. 10.2 months, P=0.075, Figure 4) in two group showed no significant difference.
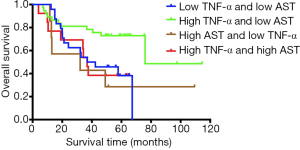
Based on the high and low levels of TNF-α and AST, we divided 81 patients with HCC into four groups for Kaplan-Meier analysis. The median OS (37 vs. 76 vs. 32.1 vs. 36.8 months, P=0.03, Figure 5) in four groups showed that patients with high expression of TNF-α and low-level AST had the longest survival time compared with other groups. The difference was statistically significant.
Univariate and multivariate Cox proportional hazard analysis
Factors that may affect patients’ postoperative recurrence and survival were included in the Cox proportional hazard model for univariate and multivariate analysis. The results of multivariate analysis showed that AST level (HR 2.251, 95% CI, 1.135–4.463, P=0.020, Table 2) and differentiation level (HR 2.518, 95% CI, 1.274–4.977, P=0.008, Table 2) were independent risk factors affecting overall patient survival. The expression levels of TNF-α and NF-κB (HR 0.438, 95% CI, 0.221–0.865, P=0.018, Table 2) was independent prognostic factors affecting overall patient survival after surgery. Similarly, tumour diameter (HR 3.117, 95% CI, 1.716–5.659, P<0.001, Table 3) and microvascular tumor thrombus (HR 2.350, 95% CI, 1.229–4.493, P=0.01, Table 3) were independent risk factors for postoperative recurrence, and PLT level (HR 0.499, 95% CI, 0.277–0.897, P=0.02, Table 3) and expression levels of TNF-α and NF-κB (HR 0.569, 95% CI, 0.334–0.970, P=0.038, Table 3) were independent prognostic factor affecting postoperative recurrence.
Table 2
| Variable | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Gender (female vs. male) | 0.751 (0.314–1.792) | 0.518 | – | – | |
| Age (y, <50 vs. ≥50) | 1.048 (0.508–2.160) | 0.899 | – | – | |
| Alcohol abuse | 1.240 (0.628–2.449) | 0.536 | – | – | |
| Tumor size (cm, <5 vs. ≥5) | 1.812 (0.915–3.588) | 0.088 | – | – | |
| Tumor margin (cm, <5 vs. ≥5) | 0.346 (0.083–1.439) | 0.144 | – | – | |
| Differentiation (high vs. middle and low) | 2.268 (1.157–4.445) | 0.017* | 2.518 (1.274–4.977) | 0.008* | |
| Microvascular tumor thrombus (yes vs. no) | 1.867 (0.883–3.947) | 0.102 | – | – | |
| Liver cirrhosis (yes vs. no) | 1.604 (0.624–4.123) | 0.327 | – | – | |
| Capsule invasion (yes vs. no) | 0.853 (0.262–2.781) | 0.793 | – | – | |
| AFP level (ng/L, ≤400 vs. >400) | 0.903 (0.427–1.910) | 0.790 | – | – | |
| ALB level (g/L, ≤35 vs. >35) | 1.755 (0.537–5.740) | 0.352 | – | – | |
| ALT level (U/L, ≤60 vs. >60) | 1.383 (0.656–2.918) | 0.395 | – | – | |
| AST level (U/L, ≤42 vs. >42) | 1.897 (0.973–3.699) | 0.060 | 2.251 (1.135–4.463) | 0.020* | |
| PLT level (104/μL, ≤10 vs. >10) | 0.505 (0.255–1.004) | 0.051 | – | – | |
| TBIL level (μmol/L, ≤22 vs. >22) | 1.646 (0.720–3.763) | 0.237 | – | – | |
| TNF-α and NF-κB (low vs. high) | 0.507 (0.260–0.988) | 0.046* | 0.438 (0.221–0.865) | 0.018* | |
*, P<0.05, statistically significant. HR, hazard ratio; CI, confidence interval; TNF-α, tumor necrosis factor-alpha; NF-κB, nuclear factor-kappa B; TBIL, total bilirubin; ALB, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; PLT, platelet; AFP, α-fetoprotein.
Table 3
| Variable | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Gender (female vs. male) | 0.663 (0.335–1.310) | 0.237 | – | – | |
| Age (y, <50 vs. ≥50) | 1.079 (0.592–1.967) | 0.804 | – | – | |
| Alcohol abuse | 1.353 (0.775–2.364) | 0.288 | – | – | |
| Tumor size (cm, <5 vs. ≥5) | 2.441 (1.387–4.297) | 0.002* | 3.117 (1.716–5.659) | <0.001* | |
| Tumor margin (cm, <5 vs. ≥5) | 0.376 (0.136–1.044) | 0.06 | – | – | |
| Differentiation (high vs. middle and low) | 1.784 (0.990–3.215) | 0.054 | – | – | |
| Microvascular tumor thrombus (yes vs. no) | 1.808 (0.975–3.352) | 0.060 | 2.350 (1.229–4.493) | 0.01* | |
| Liver cirrhosis (yes vs. no) | 1.121 (0.509–2.470) | 0.777 | – | – | |
| Capsule invasion (yes vs. no) | 1.052 (0.380–2.910) | 0.922 | – | – | |
| AFP level (ng/L, ≤400 vs. >400) | 1.216 (0.677–2.187) | 0.513 | – | – | |
| ALB level (g/L, ≤35 vs. >35) | 1.788 (0.714–4.479) | 0.215 | – | – | |
| ALT level (U/L, ≤60 vs. >60) | 1.548 (0.819–2.928) | 0.178 | – | – | |
| AST level (U/L, ≤42 vs. >42) | 1.688 (0.946–3.012) | 0.076 | – | – | |
| PLT level (104/μL, ≤10 vs. >10) | 0.536 (0.301–0.955) | 0.034* | 0.499 (0.277–0.897) | 0.02* | |
| TBIL level (μmol/L, ≤22 vs. >22) | 1.816 (0.917–3.593) | 0.087 | – | – | |
| TNF-α and NF-κB (low vs. high) | 0.625(0.370–1.056) | 0.079 | 0.569 (0.334–0.970) | 0.038* | |
*, P<0.05, statistically significant. HR, hazard ratio; CI, confidence interval; TNF-α, tumor necrosis factor-alpha; NF-κB, nuclear factor-kappa B; TBIL, total bilirubin; ALB, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; PLT, platelet; AFP, α-fetoprotein.
Discussion
Most patients with HCC are asymptomatic in the early stages and have reached an advanced stage or metastasis at the time of diagnosis. The effects of surgery, radiotherapy and chemotherapy are not significant at that stage. In recent years, an increasing number of studies have shown that inflammatory factors are inseparable from the development of HCC (17-19).
In the current research, we demonstrated that patients with high expression of TNF-α and NF-κB in HCC tissues had longer survival times than those with low expression of TNF-α and NF-κB. The survival difference between these two groups was statistically significant (P<0.05, Figure 3). TNF-α has been demonstrated to be an anti-tumour cytokine and to activate NF-κB formation (20,21). The HepG2 cell line, when treated with miRNA-491_5p, showed sensitivity to TNF-α and increased apoptosis (22). Interestingly, studies have shown that transfection of the human TNF-α gene into mice results in a significant increase in the number of hepatic metastases, demonstrating higher metastatic potential and mortality (23,24). These studies have shown a close relationship between TNF-α and NF-κB and confirmed that TNF-α plays a dual role in the development of tumours. Multidrug resistance 2 (Mdr2) gene knockout mice inhibit the progression of HCC by inhibiting TNF-α or NF-κB in the late stage of tumour development (15). Related studies have also shown that the elimination of the IkappaB kinase (IKK) subunit NF-κB essential modulator (NEMO, also known as IKKγ) can prevent the activation of NF-κB, thereby preventing tumour progression (25). Moreover, NF-κB is over-expressed in primary HCC tissues, and its level there is significantly different to that in normal liver tissues adjacent to the cancer (26). These studies mainly indicate the anti-tumour apoptosis effect of NF-κB. Although both TNF-α and NF-κB can promote tumour progression, based on our analysis, patients with high expression of TNF-α and NF-κB have longer survival times. This effect may occur because high expression of TNF-α and NF-κB is associated with the activation of certain anti-tumour pathways. The mechanism of action between TNF-α and NF-κB is not fully understood. thus, research addressing inflammatory factors in the tumour microenvironment of HCC requires further exploration.
Our study showed that high expression of TNF-α and NF-κB in the microenvironment of HCC patients was an independent prognostic factor affecting postoperative survival (HR =0.438, P=0.018, Table 2) and recurrence (HR =0.569, P=0.038, Table 3). Although studies have shown the effects of TNF-α and NF-κB in tumours (12,26), no relevant studies have previously reported on such phenomena in HCC, and through our research, patients with high expression of TNF-α and NF-κB have been shown to have longer survival times. This result corresponds to our conclusion that high expression of TNF-α and NF-κB is an independent prognostic factor affecting postoperative survival and recurrence in patients. Moreover, we believe that further study of the expression and mechanism of TNF-α and NF-κB in the microenvironment of HCC can provide a new method of treatment for HCC patients.
Related studies have shown that AST levels can be an independent risk factor for HCC (27), elevated AST levels are significantly associated with HCC (28), and AST levels are associated with HCC progression, with higher levels corresponding to more advanced progression (29). Our study also showed that high expression of AST is an independent risk factor for postoperative survival in patients with HCC (HR =2.251, P=0.02, Table 2), and the risk of postoperative death in patients with high AST expression is 2.251 times higher than that of patients with low expression. Serum AST is released from damaged hepatocytes into the blood, and their activity has been widely recognized as a marker for the detection of hepatitis, which further suggests the importance of inflammatory factors in HCC microenvironment. Therefore, we suggest that AST can be used not only as a monitoring indicator for early HCC but also early treatment to reduce the degree of tumour malignancy. The regulation of AST after surgery is also a means to prevent recurrence and improve patient survival time. Studies have also shown that injection of anti-tumour drugs into animals with induced HCC can cause a linear increase in serum TNF-α and a dose-dependent decrease in serum AST concentrations (30), which is consistent with our findings. Both high TNF-α and low AST levels are beneficial for postoperative patients. In addition, the treatment of an HCC rat model with β-carotene can lead to increased levels of TNF-α and decreased AST levels in rats (31), which further confirms our conclusion. We divided 81 patients with BCLC-0-B HCC into four groups according to the high and low levels of TNF-α and AST. The effects of TNF-α and AST on the prognosis of patients were further analyzed. The results showed high expression of TNF-α and low-level AST patients with HCC have the best survival time, and the difference was statistically significant (P=0.03, Figure 5), which further validates our conclusions, and we will go deeper into the study of inflammatory factors in future studies.
The inflammatory factors in the microenvironment of HCC play an important role in the development of HCC. We have investigated the inflammatory factors in the microenvironment of HCC to prove that high expression of TNF-α and NF-κB can confer longer patient survival time. In subsequent studies, we will increase the sample size to further confirm the relevant conclusions and further explore the mechanism by which inflammatory factors affect tumour development.
Conclusions
According to our research, the expression of TNF-α and NF-κB in the HCC microenvironment is related to postoperative survival, and high expression of TNF-α and NF-κB can prolong patient survival time. This study can provide some guidance and treatment advice for postoperative survival and recurrence in HCC patients.
Acknowledgments
The research and writing of this thesis were completed under the careful guidance of my teacher and maoge. I would like to extend my sincere gratitude and lofty respect to them.
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.03.09). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study and related use of the patients’ samples were approved by The Ethics Committee of the Affiliated Hospital of Qingdao University, and written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gao J, Xie L, Yang WS, et al. Risk factors of hepatocellular carcinoma--current status and perspectives. Asian Pac J Cancer Prev 2012;13:743-52. [Crossref] [PubMed]
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Macewan DJ. TNF receptor subtype signalling: Differences and cellular consequences. Cell Signal 2002;14:477-92. [Crossref] [PubMed]
- Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ 2003;10:45-65. [Crossref] [PubMed]
- Chen G, Goeddel DV. TNF-R1 signaling: a beautiful pathway. Science 2002;296:1634-5. [Crossref] [PubMed]
- Micheau O, Tschopp J. Induction of TNF Receptor I-Mediated Apoptosis via Two Sequential Signaling Complexes. Cell 2003;114:181-90. [Crossref] [PubMed]
- Landskron G. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res 2014;2014:149185. [Crossref] [PubMed]
- Xiang CY, He XY, Li ZB, et al. Recent advance in the role of TNF-α in cancer. Chinese Bulletin of Life Sciences 2012;24:250-4.
- Horssen RV, Hagen TL, Eggermont AM. TNF-α in Cancer Treatment: Molecular Insights, Antitumor Effects, and Clinical Utility. Oncologist 2006;11:397-408. [Crossref] [PubMed]
- Kulbe H, Thompson R, Wilson JL, et al. The Inflammatory Cytokine Tumor Necrosis Factor-α Generates an Autocrine Tumor-Promoting Network in Epithelial Ovarian Cancer Cells. Cancer Res 2007;67:585-92. [Crossref] [PubMed]
- Lee CY, Hsu YC, Wang JY, et al. Chemopreventive effect of selenium and Chinese medicinal herbs on N‐nitrosobis(2‐oxopropyl)amine‐induced hepatocellular carcinoma in Syrian hamsters. Liver Int 2008;28:841-55. [Crossref] [PubMed]
- Fredriksson L, Herpers B, Benedetti G, et al. Diclofenac inhibits tumor necrosis factor‐α‐induced nuclear factor‐κB activation causing synergistic hepatocyte apoptosis. Hepatology 2011;53:2027-41. [Crossref] [PubMed]
- Knight B, Yeoh GCT, Husk KL, et al. Impaired Preneoplastic Changes and Liver Tumor Formation in Tumor Necrosis Factor Receptor Type 1 Knockout Mice. J Exp Med 2000;192:1809-18. [Crossref] [PubMed]
- Pikarsky E, Porat RM, Stein I, et al. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature 2004;431:461-6. [Crossref] [PubMed]
- Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell 2002;109:S81-96. [Crossref] [PubMed]
- Capece D, Fischietti M, Verzella D, et al. The inflammatory microenvironment in hepatocellular carcinoma: a pivotal role for tumor-associated macrophages. Biomed Res Int 2013;2013:187204. [Crossref] [PubMed]
- Jang MK, Kim HS, Chung YH. Clinical aspects of tumor necrosis factor-α signaling in hepatocellular carcinoma. Curr Pharm Des 2014;20:2799-808. [Crossref] [PubMed]
- Leonardi GC, Candido S, Cervello M, et al. The tumor microenvironment in hepatocellular carcinoma Int J Oncol 2012;40:1733. (review). [PubMed]
- Old L. Tumor necrosis factor (TNF). Science 1985;230:630-2. [Crossref] [PubMed]
- Finco TS, Beg AA, Baldwin AS. Inducible phosphorylation of I kappa B alpha is not sufficient for its dissociation from NF-kappa B and is inhibited by protease inhibitors. Proc Natl Acad Sci U S A 1994;91:11884-8. [Crossref] [PubMed]
- Yoon S, Kim TH, Natarajan A, et al. Acute liver injury upregulates microRNA‐491–5p in mice, and its overexpression sensitizes Hep G2 cells for tumour necrosis factor‐α‐induced apoptosis. Liver Int 2010;30:376-87. [Crossref] [PubMed]
- Orosz P, Krüger A, Hubbe M, et al. Promotion of experimental liver metastasis by tumor necrosis factor. Int J Cancer 1995;60:867. [Crossref] [PubMed]
- Malik ST, Naylor MS, East N, et al. Cells secreting tumour necrosis factor show enhanced metastasis in nude mice. Eur J Cancer 1990;26:1031-4. [Crossref] [PubMed]
- Luedde T, Beraza N, Kotsikoris V, et al. Deletion of NEMO/IKKγ in Liver Parenchymal Cells Causes Steatohepatitis and Hepatocellular Carcinoma. Cancer Cell 2007;11:119-32. [Crossref] [PubMed]
- Zhao B, Li HQ, Li GS, et al. Role of nuclear factor-κB pathway and TNF alpha and intercellular adhesion molecule-1 in primary hepatocellular carcinoma. China Journal of Modern Medicine 2014;13.
- Wen CP, Lin J, Yang YC, et al. Hepatocellular Carcinoma Risk Prediction Model for the General Population: The Predictive Power of Transaminases. J Natl Cancer Inst 2012;104:1599. [Crossref] [PubMed]
- Bayomi EA, Barakat AB, El-Bassuoni MA, et al. Cyclooxygenase-2 expression is associated with elevated aspartate aminotransferase level in hepatocellular carcinoma. J Cancer Res Ther 2015;11:786-92. [Crossref] [PubMed]
- Nirei K, Matsuoka S, Nakamura H, et al. Incidence of hepatocellular carcinoma reduced by phlebotomy treatment in patients with chronic hepatitis C. Intern Med 2015;54:107. [Crossref] [PubMed]
- Chen B, Zhou W, Ning M, et al. Evaluation of antitumour activity of tea carbohydrate polymers in hepatocellular carcinoma animals. Int J Biol Macromol 2012;50:1103-8. [Crossref] [PubMed]
- Cui B, Liu S, Wang Q, et al. Effect of β-carotene on immunity function and tumour growth in hepatocellular carcinoma rats. Molecules 2012;17:8595. [Crossref] [PubMed]