The role of magnetic resonance imaging in detection and surgical treatment of breast intraductal papillomas
Introduction
Breast intraductal papilloma/papillomatos, which includes solitary intraductal papilloma, multiple intraductal papilloma(s) and intraductal papillomatosis, is a group of common breast epithelial benign tumors (1). Most patients do not have clinically palpable tumors, whether a nipple charge is present or not, and these tumors are untouchable, small or multi-focal. Clinical research has shown that approximately 11% of papillomas can develop into malignant diseases. The intraductal papillomatosis, particularly, has malignancy rate of up to 30% (2-4). Therefore, aggressive surgery is required to treat against the risk of a subsequent carcinoma.
However, diagnosis requires a complete clinical history, physical examination, cytological examination and imageological examination (5). Unfortunately, the clinical history and physical and cytological examination are not always diagnostic. Mammography, galactography, breast duct endoscopy and ultrasound are considered traditional cytological examinations (6). Problematically, all these methods have limitations or defects, which can lead to a lack of clarity in pre-surgery diagnosis.
The treatment of breast intraductal papillomatosis is controversial and the current consensus is segmental adenectomy of the lesion duct (7). Radical operation will undergo when the patients are suspicious of malignance. The principle of treatment is a completely resection without residual which may leads to recurrence. The key of completely resection is evaluating disease extent and lesions’ pre-surgery (8). Traditional pre-surgery evaluation (galactography breast duct endoscopy) cannot assess the range of lesions due to the tumors are mostly small or multiple. And traditional surgery methods are ultrasound combined with clinical examination and methylene blue notation excision. However, ultrasound combined with clinical examination show lesions uncomprehensive and the role of guidance is too weak to display internal pathological changes, especially for small, deep, flake, scattered or multiple intraductal lesions. Methylene blue notation just applies to the patients with nipple discharge pre-surgery.
Magnetic resonance imaging has received much attention in recent years due to its high contrast resolution properties in comparison to the conventional imageological examinations (9,10). There are three sequences and three-dimensional (3D) positionings which can comprehensively display the entire lesion, scope and the relationship with the surroundings in pre-surgery or surgery (11). Furthermore, MRI is highly sensitive in detecting breast disease such as invasive breast cancer (60–100%) and DCIS (77–96%) (12). Recently, several studies have been conducted to evaluate the potential MRI diagnostic role in breast intraductal papilloma/papillomatosis (13,14), but few researchers have made a comparison between using MRI scanning technique, and conventional image modalities to completely identify the location of lesions pre-surgery and during surgery.
To improve diagnosis outcomes in breast papillomatosis patients, the aim of this study is to comparatively evaluate MRI imaging versus conventional method ultrasound, in terms of the diagnosis role, definition accuracy of lesions’ location and the guidance of resection, both pre-surgery and in surgery.
Methods
Patients
A retrospective analysis of the patients was carried out, who were confirmed by surgery pathology for solitary intraductal papilloma, multiple intraductal papilloma and intraductal papillomatosis disease in the People’s Liberation Army General Hospital from January 2012 to September 2017. A total of 245 patients were found who underwent both consecutive breast MRI and ultrasound examination. The usage of these images and the patients’ information were approved by the Ethics Committee of the PLA Hospital.
MRI
MRI scan was performed using a 1.5-T magnet (Magnetom Avanto, Siemens Medical Solution, Erlangen, Germany) with a dedicated bilateral breast surface coil using the prone position.
The image protocol and parameters were as follows: using an axial T1-weighted image (TR/TE, 500/11) and a T2-weighted turbo spin-echo image (5,000/100) of both of the breasts to obtain a 3 mm slice thickness. T1-weighted images were acquired using a 3D FLASH (fast low-angle shot pulse sequence) with a fat-selective inversion for fat suppression through both of the breasts (TR/TE 3.91/1.42, flip angle 300). Pre-contrast images were obtained over a 512×317 matrix in the axial plane with a slice thickness of 1.5 mm without the gap before the administration of a contrast agent. Contrast-enhanced dynamic imaging was performed with an injection of gadopentetate dimeglumine (Magnevist, Berlex Laboratories, Wayne, NJ, USA) at a rate of 2 mL/s via an automatic injector at a dose of 0.1–0.2 mmol/L per kilogram of body weight, together with a 10-mL bolus of saline solution. Five sequential contrast-enhanced images were acquired at every 1 min interval. The pre-contrast images were then subtracted from the corresponding postcontrast images on a pixel-by-pixel basis with use of the standard software subtraction function available on our console.
The MRI images were analyzed with a software named GE ADW 4.3 workstation for image interpretation. Reconstruct sagittal and coronal image by 3D-FSPGR T1WI-FS transverse dynamic enhanced scan. The largest lesions and/or the most significant parts were selected as the interested area. The morphologic characteristics of the lesions were identified, then the lesion characteristics were catalogued according to standards set by the American College of Radiology’s Breast Image Reporting and Data System (BI-RADS) lexicon. For the analysis of enhancement kinetics, time-intensity curves (TIC) were plotted from the signal intensity values obtained from the most enhanced part of the lesion. According to the phase analysis of peak enhancement and washout of contrast enhancement, TIC profile was also classified into the following 3 types: (I) type I persistent, characterized by initial increase in signal intensity followed by more than 10% increase in signal intensity in the delayed phase; (II) type II plateau, characterized by initial rapid increase rise in signal intensity, followed by less than 10% change in signal intensity in the delayed phase, with enhancement increased in the early phase and followed by a plateau; (III) type III washout, characterized by initial increase in signal intensity followed by more than 10% reduction in signal intensity in the delayed phase, with peak enhancement seen within 120 s (early phase) and followed by a decrease.
MRI examination was retrospectively and independently reviewed by two radiologists who have experience in interpreting breast MRI examinations. The common MRI positive findings were then approved when in regards to the terms of BI-RADS 3–5.
Ultrasound examination
The ultrasound was performed using Siemens Sequoia 512 ultrasonic diagnosis instrument with 15L-8 probe and Philips iU22 12–5 probes. Based on the presence of solid focally with or without duct dilated, intraductal papilloma (disease), performance of ultrasonic images can be divided into the following three types: (I) solid nodules type without duct dilated (low parenchyma echo nodules); (II) duct dilated areola; (III) hybrid type (duct dilated with nodules). The BI-RADS hierarchical diagnostic analysis was carried out on the lesions. Ultrasonic positive was defined as long as three types occurred. An ultrasound doctor operated the ultrasound examination, and two doctors performed the ultrasound imaging description.
Operation methods
The surgical incision consisted of two types: arc incision and radial incision. The arc incision surrounding with mammary areola where are a lot of wrinkles and this part of skin is darker, so that the incision is hidden and does not affect the appearance. On the other hand, in order not to damage the mammary duct, a radial incision parallel to the skin texture and perpendicular to dizziness was chosen.
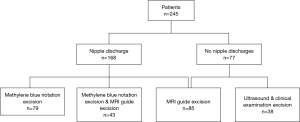
The lesions in this study were operated on by both surgery and excision biopsy (and not endoscopic or percutaneous types). Patients with nipple discharge were injected with methylthionine as guidance. Therefore, patients were assigned to the four groups depending on nipple discharge. If there was nipple discharge, the patients were treated with surgery by simple methylene blue excision, simple MRI guide excision and methylene blue & MRI cooperative surgery. If there was no nipple discharge, the patients were treated with MRI guide excision or the traditional method of surgery under the guidance of clinical examination & ultrasound imaging.
- Ultrasound & clinical examination excision: based on clinical examination and ultrasonic image, surgeon infers the proposed excision lesion and designs the incision size pre-surgery. Then, local infiltrate of 1% lidocaine, excise the skin and subcutaneous tissue, looking for visible diseased tissue and corresponding section of diseased duct and its disposal. The operation depends on surgeon’s clinical experience, then send frozen to pathological examination immediately;
- Methylene blue notation excision: Patients are placed in a supine position and extrude the breast tissue all round of the nipple. Then, ball back needle along the breast tube was inserted to the discharge pipe and inject a little methylene blue (about 0.2–0.3 mL, the injection rate will be subject to slight overflow dyeing agent) shortly after discharging the fluid milk tube completely. Next, remove the syringe in breast duct. Eventually, contort the direction on the basis of discharge tube position and figure out the lesion location as well as selecting the incision areola. Next, cut the skin and subcutaneous tissue and looking for the blue dye expansion tube and pull out the needle and find blue staining sections. Finally, resect specimens along with the blue edge of tissue. Remove the visible diseased tissue which was not blue dye in surgery and send samples to frozen pathological examination;
- Methylene blue notation & MRI excision: patients are placed in a supine position. Design incision based on MRI images shown the lesion location and scope pre-surgery. Then undergo surgery combing with the methylene blue method (like the above procedure);
- MRI guide excision: patients are placed in a supine position, and the position of the lesion is estimated according to the MRI transverse, coronal, sagittal and 3D images. Resection size and appropriate incision is designed, along with the nipples and skin lesion location. According to the results of 3D image positioning, the skin and subcutaneous tissue is cut, particularly visibly diseased tissue and corresponding section of diseased duct and its disposal. then follow the steps the same as above;
- All the surgery was operated by one and the same doctor. Then 3-years follow-up for recurrence was conducted after surgery, with the follow-up deadline on July 1, 2017. The median follow-up time was 46 months.
Statistical analysis
Measurement data shows mean ± standard deviation (mean ± SD). This was based on the quantitative data using t-test statistical analysis, the qualitative data statistical analysis model, as well as using the Chi square test, and the Fisher’s exact test. All statistical analysis was performed using SPSS 19.0 statistical software and all results with P<0.05 were considered significant.
Results
All patients were women, aged between 22 and 85 years (with a median age of 46 years). All the patients were operated on the hospital by one surgeon.
Clinical characteristics of patients
A total of 161 and 48 patients separately had a pathological diagnosis of solitary and multiple papilloma, with 31 and 12 having concomitant atypical ductal hyperplasia (ADH), respectively; 36 patients had papillomatosis, 14 of these had ADH, 3 had invasive tumors and 2 had ductal carcinoma in situ (DCIS). As can be seen in Table 1, solitary papilloma patients present with nipple discharge more than 50%, and as few as 14.3% symptomatic present with breast mass. In addition, mass was most commonly observed in symptomatic multiple papilloma patients, similar with papillomatosis. Nearly one-third of the patients who were present were discharged had palpable lumps, in 41/161 (25.5%) of the patient group, 18/48 (37.5%), and 11/36 (30.6%) of the patients’ group separately, combined. A few patients were asymptomatic with no palpable lumps.
Table 1
| Characteristics | Solitary papilloma (n=161), n (%) | Multiple papilloma (n=48), n (%) | Papillomatosis (n=36), n (%) |
|---|---|---|---|
| Discharge | 87 (54.0) | 7 (14.6) | 5 (13.9) |
| Lump | 23 (14.3) | 19 (39.6) | 17 (47.2) |
| Discharge & lump | 41 (25.5) | 18 (37.5) | 11 (30.6) |
| Asymptomatic | 10 (6.2) | 3 (6.3) | 3 (8.3) |
Detection and initial definition of lesions pre-surgery compared MRI with ultrasound image
All 245 patients underwent an ultrasound and MRI examination, and 168 of the 243 cases with positive signs were detected, with detection rate being 65.4% and 99.0%, respectively. It is clear that MRI has an extremely higher detection rate when it is compared with ultrasound.
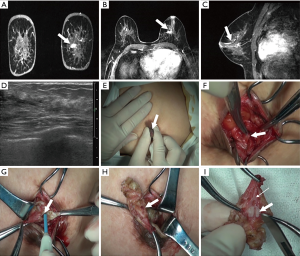
MRI imaging shown the morphology of all the lesions without omissions, including the feature of shape, border and contrast pattern (Table 2). According to the characteristics of MRI imaging, lesions can be divided into mass-like lesions (including solitary and multiple, Figure 1A,B) and non-cancerous seeming lesions (including ductal and regional, Figure 1C,D). The characteristic shape of mass lesions is round, lobulated, irregular and the border is well-defined, microlobulated, indistinct and the internal enhancement is homogeneous, heterogeneous, rim enhancement. On the other hand, the non-mass lesions have various shapes such as ductal, segmental, regional, multiple mass-like, clustered ring enhancement, and focal nodulary-punctate. However, it is apparent that in the majority of cases that lesions were just present in the form of a mass or masses in ultrasound image (Figure 1E,F).
Table 2
| Morphological description | Solitary papilloma (n=166), n (%) | Multiple papilloma (n=46), n (%) | Papillomatosis (n=33), n (%) |
|---|---|---|---|
| Shape | |||
| Round | 44 (26.5) | 14 (30.4) | 6 (18.2) |
| Lobulated | 51 (30.7) | 14 (30.4) | 6 (18.2) |
| Irregular | 22 (13.3) | 6 (13.0) | 5 (15.2) |
| Border | |||
| Well defined | 81 (48.8) | 18 (39.1) | 10 (30.3) |
| Micro lobulated | 10 (6.0) | 5 (10.9) | 3 (9.1) |
| Indistinct | 13 (7.8) | 8 (17.4) | 7 (21.2) |
| Contrast pattern | |||
| Homogeneous | 69 (41.6) | 16 (34.8) | 9 (27.3) |
| Heterogeneous | 16 (9.6) | 6 (13.0) | 4 (12.1) |
| Rim enhancement | 13 (7.8) | 6 (13.0) | 8 (24.2) |
| Nonmass-like | |||
| Ductal | 22 (13.3) | 4 (8.7) | 4 (12.1) |
| Segmental | 37 (22.3) | 2 (4.3) | 7 (21.2) |
| Regional | 4 (2.4) | 2 (4.3) | 2 (6.1) |
| Multiple mass-like | 2 (1.2) | 6 (13.0) | 6 (18.2) |
| Clustered ring enhancement | 0 (0.0) | 3 (6.5) | 2 (6.1) |
| Focal nodulary-punctate | 0 (0.0) | 2 (4.3) | 5 (15.2) |
| Dynamic contrast enhancement | |||
| Type I persistent | 55 (33.1) | 8 (17.4) | 8 (24.2) |
| Type II plateau | 42 (25.3) | 9 (19.6) | 4 (12.1) |
| Type III washout | 16 (9.6) | 4 (8.7) | 4 (12.1) |
| Nonmass-like enhancement | 39 (23.5) | 14 (30.4) | 24 (72.7) |
| Mass & nonmass-like enhancement | 20 (12.0) | 6 (13.0) | 12 (36.4) |
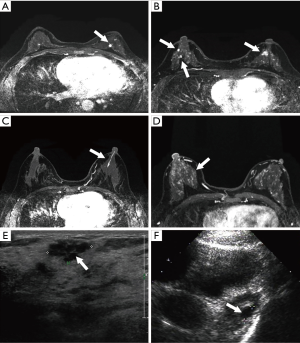
MRI image could comprehensively locate the lesions pre-surgery and guide an excision
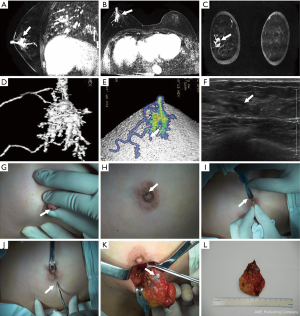
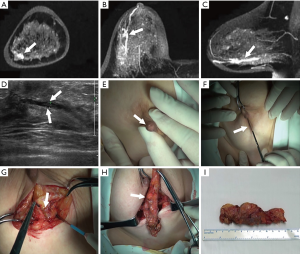
All the patients underwent surgical resection. Patients with no nipple discharge were surgically excised under guidance of ultrasonic & clinical examination (conventional operation method) or MRI image. On the other hand, patients with nipple discharge were excision treated relying on methylene blue notation (conventional operation method) or methylene blue notation & MRI or MRI image guide excision (Figure 2). MRI image could comprehensively show the lesions’ shape and location, and thus could guide the excision in surgery (Figures 3-7).


Traditional surgery methods are more likely to relapse compared with MRI guide groups
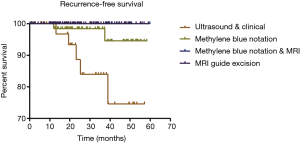
A total of 238 patients attended follow-up after surgery and the recurrence-free survival rate was analyzed (Figure 8); 5/39 patients relapsed in the ultrasonic & clinical examination excision group. Among them, 2 patients were multiple papilloma and 3 cases were papillomatosis. Moreover, there were 2 patients who had papillomatosis that relapsed in the methylene blue notation group (79 patients). No one relapsed in the MRI guide groups. It is very likely that the residual in surgery is one of the main causes for the postoperative recurrence.
Discussion
Although intraductal papilloma is uncommon, it is the most frequently encountered benign papillary lesion in the breast. Solitary intraductal papilloma mainly occurs in big catheters, and is often accompanied by a bloody nipple discharge, with malignant being uncommon as a whole (17). However, multiple papilloma and papillomatosis that originate in the small or terminal catheter, generally show breast neoplasm symptoms without a nipple(s) discharge and the rates of canceration are higher as reported. In this study, we show that solitary intraductal papilloma is one of the most common types (accounting for 65.7% and 54% with nipple discharge). Conversely, multiple papilloma and papillomatosis is much less common, 19.6% and 14.7% respectively and mass symptoms is in the majority (18). The overall response is consistent with the literature reported.
Conventional imageological examinations for papilloma lesions includes, galactography, mammary ductoscopy and ultrasound. Although the principles and techniques of various imaging methods are different, all of these examination types have their own limitations and shortcomings (19). Galactography and mammary ductoscopy can observe lesions directly but they are unable to comprehensively display the complete lesion or lesions. Additionally, these methods are invasive, and as a result, the nipple discharge may be stopped, causing a hemorrhage of the breast, effusion, or even difficulties in finding the discharge duct and the subordinate section during the surgery (5). Ultrasound responds to the difference of echoes coming from various organs and lesions; however, it is difficult to distinguish the non-cancerous seeming lesions and normal breast tissue (20). Our hospital does not to use galactography and mammary ductoscopy examinations; therefore, there is no comparison between them and MRI in this study.
MRI has a highly sensitivity (70–100%) in diagnosis of mammary intraductal papilloma (disease). Compared to previous studies, 21 cases of intraductal papilloma (disease) were retrospectively analyzed in MRI detection rate were 100% (11). The study of 31 cases of intraductal papilloma were retrospectively analyzed in MRI detection rate of 93.5%. In this study, the rate of MRI detection is 99.0%. Despite the various research methods perhaps being slightly different, the results are similar (21), all which demonstrates that MRI has a high detection rate in intraductal papilloma.
In addition, MRI image can also display various forms of intraductal papilloma, including mass-like and non-mass-like types, along with concreteness categories consisting of solitary mass-like lesions, multiple mass-like lesions, ductal nonmass-like lesions and regional nonmass-lesions. Nevertheless, ultrasonic image mainly characterized as duct expansion, solid mass, mixed type (solid mass with duct expansion) and no abnormal echo (22). It is clear that mass can be present in MRI and ultrasound, but MRI can also display the nonmass-like lesions. Moreover, MRI could clearly show the relationship between lesions and ducts even in sample thickness of 1.2 mm or less (usually <3 mm). It can be said that MRI can find its target as long as there is an abnormal lesion. Furthermore, MRI shows bilateral breast images with highly spatial resolution, including the morphological features of lesions, internal reinforcement, and even bilateral multiple and small lesions, which could be reconstructed and observed with any direction (23,24).
The treatment of papilloma as the disease, is given MRI positive priority to surgery, which can be guided by traditional ultrasound image and methylene blue notation. However, there are many problems and hidden dangers. Usually, ultrasound can’t clearly show the range of lesions, especially when the lesions are in a deep position, or close to the chest wall, and even if the tumor is very small or non-mass-like. It seems that the surgical excision depends on the ultrasound image and the clinical examination is blind even though surgeon can successfully find lesions. Methylene blue notation surgery is another conventional method but is regrettably impractical because many patients appear with no nipple discharge symptoms. In addition, methylene blue is unable to flow into the lower laticifer if the dominant tube papilloma leading to the duct is blocked. It is obvious that just excising spelling tissues is not enough and it’s very likely to relapse.
One of the advantages of MRI in guiding excisions is that MRI images can clearly display the duct shape, mass lesions position, as well as the actual lesion (25,26). As this study shows in Figures 4,5, a surgeon can accurately evaluate the lesions’ location, range and catheter pre-surgery and then design the most appropriate incision by reading the MRI image (transverse, coronal and sagittal three axis), including the 3D image. Depending on this data, the surgeon can make a better location of incision and find lesions successfully, so as to avoid cutting too much glandular tissue lesions, and thus achieving an elegant appearance through a reduction of trauma.
Another benefit of MRI in operation guidance lies in the fact that surgeons can fortunately perform the surgery and guided by MRI imaging but also for patients with no nipple discharge or discharge stopped pre-surgery, or in whom ultrasound found no abnormal lesions, or asymptomatic.
The key to successful surgery is a complete resection of the lesions. It is difficult to find and remove lesions completely by depending on clinical examination as some lesions are small and multiple. In this study, there were 7 cases of recurrence, which all underwent conventional treatment methods, with the traditional surgery groups having a shorter recurrence survival time. This research still cannot state conclusively that MRI guidance can reduce recurrence because follow-up time is short. However, it is still a possibility that some lesions have not been completely removed in surgery (27).
Some limitations of our study include its retrospective design and inability to carry out intraductal image techniques such as ductoscopy or galactography in all cases. In addition, it would be better if the follow-up time was longer.
In conclusion, MRI is an effective imaging tool in preoperatively detecting and defining intraductal papilloma disease, in addition to accurately locating the lesions in pre-surgery and precisely guiding excision in surgery. MRI can be widely used in clinical breast intraductal papilloma.
Conclusions
MRI is accurate at the detection, localization and resection range of intraductal papilloma lesions, which is very helpful in breast surgery.
Acknowledgments
We thank department of pathology and medical image of the Peoples Liberation Army General Hospital for their support to this study. The authors take full responsibility for analyses and interpretation of these cancer registry data.
Funding:
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.03.27). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the PLA Hospital (IRB number: SQ/01.01/01.3) and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Choi SH, Jo S, Kim DH, et al. Clinical and imaging characteristics of papillary neoplasms of the breast associated with malignancy: a retrospective cohort study. Ultrasound Med Biol 2014;40:2599-608. [Crossref] [PubMed]
- Lewis JT, Hartmann LC, Vierkant RA, et al. An analysis of breast cancer risk in women with single, multiple, and atypical papilloma. Am J Surg Pathol 2006;30:665-72. [Crossref] [PubMed]
- Ohuchi N, Abe R, Kasai M. Possible cancerous change of intraductal papillomas of the breast. A 3-D reconstruction study of 25 cases. Cancer 1984;54:605-11. [Crossref] [PubMed]
- Page DL, Salhany KE, Jensen RA, et al. Subsequent breast carcinoma risk after biopsy with atypia in a breast papilloma. Cancer 1996;78:258-66. [Crossref] [PubMed]
- Yucesoy C, Ozturk E, Ozer Y, et al. Conventional galactography and MR contrast galactography for diagnosing nipple discharge: preliminary results. Korean J Radiol 2008;9:426-31. [Crossref] [PubMed]
- Eiada R, Chong J, Kulkarni S, et al. Papillary lesions of the breast: MRI, ultrasound, and mammographic appearances. AJR Am J Roentgenol 2012;198:264-71. [Crossref] [PubMed]
- Matsunaga T, Kawakami Y, Namba K, et al. Intraductal biopsy for diagnosis and treatment of intraductal lesions of the breast. Cancer 2004;101:2164-9. [Crossref] [PubMed]
- Kowalchik KV, Vallow LA, McDonough M, et al. The role of preoperative bilateral breast magnetic resonance imaging in patient selection for partial breast irradiation in ductal carcinoma in situ. Int J Surg Oncol 2012;2012:206342. [Crossref] [PubMed]
- Harms SE, Harms DE, Pope K, et al. Breast MR for intraductal masses. Eur J Radiol 2012;81:S59-60. [Crossref] [PubMed]
- Zhu Y, Zhang S, Liu P, et al. Solitary intraductal papillomas of the breast: MRI features and differentiation from small invasive ductal carcinomas. AJR Am J Roentgenol 2012;199:936-42. [Crossref] [PubMed]
- Son EJ, Kim EK, Kim JA, et al. Diagnostic value of 3D fast low-angle shot dynamic MRI of breast papillomas. Yonsei Med J 2009;50:838-44. [Crossref] [PubMed]
- Ballesio L, Maggi C, Savelli S, et al. Role of breast magnetic resonance imaging (MRI) in patients with unilateral nipple discharge: preliminary study. Radiol Med 2008;113:249-64. [Crossref] [PubMed]
- Sarica O, Uluc F, Tasmali D. Magnetic resonance imaging features of papillary breast lesions. Eur J Radiol 2014;83:524-30. [Crossref] [PubMed]
- Dietzel M, Baltzer PAT, Kaiser WA. Potential of MR-mammography for identification of intraductual papillomas. Eur J Radiol 2012;81:S33-4. [Crossref] [PubMed]
- Hao N, Yuan X, Wang Q, et al. Video of the surgery procedure for patient in Figure 3. Asvide 2019;5:120. Available online: http://www.asvide.com/article/view/31286
- Hao N, Yuan X, Wang Q, et al. Video of the surgery procedure for patient in Figure 4. Asvide 2019;5:121. Available online: http://www.asvide.com/article/view/31288
- Jagmohan P, Pool FJ, Putti TC, et al. Papillary lesions of the breast: imaging findings and diagnostic challenges. Diagn Interv Radiol 2013;19:471-8. [PubMed]
- Glenn ME, Throckmorton AD, Thomison JB 3rd, et al. Papillomas of the breast 15 mm or smaller: 4-year experience in a community-based dedicated breast imaging clinic. Ann Surg Oncol 2015;22:1133-9. [Crossref] [PubMed]
- Krishnamurthy S, Bevers T, Kuerer H, et al. Multidisciplinary considerations in the management of high-risk breast lesions. AJR Am J Roentgenol 2012;198:W132-40. [Crossref] [PubMed]
- Brennan SB, Corben A, Liberman L, et al. Papilloma diagnosed at MRI-guided vacuum-assisted breast biopsy: is surgical excision still warranted? AJR Am J Roentgenol 2012;199:W512-9. [Crossref] [PubMed]
- Hawley JR, Lawther H, Erdal BS, et al. Outcomes of benign breast papillomas diagnosed at image-guided vacuum-assisted core needle biopsy. Clin Imaging 2015;39:576-81. [Crossref] [PubMed]
- Xia HS, Wang X, Ding H, et al. Papillary breast lesions on contrast-enhanced ultrasound: morphological enhancement patterns and diagnostic strategy. Eur Radiol 2014;24:3178-90. [Crossref] [PubMed]
- Manganaro L, D'Ambrosio I, Gigli S, et al. Breast MRI in patients with unilateral bloody and serous-bloody nipple discharge: a comparison with galactography. Biomed Res Int 2015;2015:806368. [Crossref] [PubMed]
- Dietzel M, Kaiser C, Baltzer PA. Magnetic resonance imaging of intraductal papillomas: typical findings and differential diagnosis. J Comput Assist Tomogr 2015;39:176-84. [Crossref] [PubMed]
- Kurz KD, Roy S, Saleh A, et al. MRI features of intraductal papilloma of the breast: sheep in wolf's clothing? Acta Radiol 2011;52:264-72. [Crossref] [PubMed]
- Lorenzon M, Zuiani C, Linda A, et al. Magnetic resonance imaging in patients with nipple discharge: should we recommend it? Eur Radiol 2011;21:899-907. [Crossref] [PubMed]
- Linda A, Zuiani C, Furlan A, et al. Nonsurgical management of high-risk lesions diagnosed at core needle biopsy: can malignancy be ruled out safely with breast MRI? AJR Am J Roentgenol 2012;198:272-80. [Crossref] [PubMed]