
Cisplatin chemotherapy-induced miRNA-210 signaling inhibits hepatocellular carcinoma cell growth
Introduction
Hepatocellular carcinoma (HCC) is the fifth most prevalent type of cancer affecting the global population, with mortality rate ranking third in the world (1,2). Although successful local therapies, such as surgery or transcatheter arterial chemoembolization, have been adopted after primary diagnosis through several effective biomarkers like AFP, patients with HCC still develop a high rate of recurrence due to local invasion and intrahepatic metastasis (3). Conventional therapies using cytotoxic agents showed low response rate (4). Hepatic arterial infusion chemotherapy (HAIC) is an option for patients with HCC, which can maximize drug concentrations in the liver while keeping systemic exposure low (5).
MicroRNAs (miRNAs) are small, 20- to 24-nucleotide and non-coding RNAs found in diverse organisms and have a broad impact on gene expression through post-transcription suppression or translational repression (6,7). Previous studies indicated that deregulation of miRNAs was common in HCC tumorigenesis (8-11). For instance, miR-199a/b-3p is consistently decreased in HCC, and its decrement significantly correlates with poor survival of HCC patients (12). Another study showed that microRNA-125a-5p was significantly downregulated in 80% HCC tissues and accompanied with upregulation of several oncogenes, such as sirtuin-7, matrix metalloproteinase-11, and c-Raf (13). Recent reports also indicated specific miRNAs as prognostic factors for chemotherapy in several solid cancers (14,15). These findings suggest that miRNAs not only act as oncomiRs or tumor suppressive miRNAs but function as chemotherapeutic targets. miR-210 is involved in diverse biological and pathological processes (16,17). For instance, miR-210 modulates endothelial cell response to hypoxia by targeting ephrin A3 (EFNA3), and miR-210 can inhibit oxygen-glucose deprivation through suppression of the apoptosis of PC12 cells (18,19). The level of serum miR-210 is correlated with hepatitis B virus infection in the liver (20,21). However, the functions of miR-210 in HCC and chemotherapy remain unknown.
In this study, we showed that miR-210 expression was upregulated in HCC tissues and correlated with recurrence of HCC patients who received chemotherapy. We also demonstrated that miR-210-induced EFNA3 signaling was a therapeutic target of cisplatin in HCC cells.
Methods
Tissue samples and cell lines
This study involved samples from patients after HCC surgery, including tumor and non-tumor liver tissues. The patient characteristics can be seen in Table S1. RNA of non-cancer liver samples from at least 3 donors constituted the total RNA pool, and the detailed information for each donor was obtained from Ambion Inc. This study was approved by the ethical board of the Affiliated Tumor Hospital of Harbin Medical University.
Huh7, Bel402, and SMMC7721 cells were purchased from the Shanghai Cell Bank, Chinese Academy of Sciences. HepG2, Hep3b, and PLC cells were obtained from the American Type Culture Collection. All the cells were cultured in DMEM (Hyclone) supplied with 10% fetal bovine serum (Hyclone) at 37 °C in 5% CO2.
RNA isolation and quantitative real-time PCR (qRT-PCR) analysis
Total RNA was extracted from the fresh tissue samples and cultured cells with TRIzol (Invitrogen, Carlsbad, CA, USA). qRT-PCR analysis was performed to assess the levels of miR-210 by TaqMan probes (Invitrogen, Carlsbad, CA, USA) according to the manufacturer. Endogenous U6 snRNA was used as a control. Relative expression of miR-210 was calculated by the equation 2−ΔΔCt (ΔCT = CTmiR-210 − CTU6). The value of the relative expression ratio <1.0 was considered as low expression in the tumor relative to the non-tumorous control, whereas others were considered as high expression. Primers for qRT-PCR are shown in Table S2.
Constructs, reagents
The 3'-UTR of the human EFNA3 mRNA was cloned in pRL-TK (Promega). Mutation of the targeted sequence was created by using a QuickChange Site-Directed Mutagenesis kit (Stratagene). Sequences of primers are shown in Table S2. miR-210 and control miRNA mimic/inhibitor were purchased from Dharmcon Inc. Cisplatin was obtained from Hospira Australia Pty Ltd.
Luciferase assay
HepG2 and PLC/PRF/5 cells were seeded in 24-well plates (1×105 cells per well). When the cells grew to a density of 70% confluence, they were transfected with pRL-TK luciferase reporter plasmid (50 ng per well), pGL3-control firefly luciferase plasmid (10 ng per well). Effectene (QIAGEN) was used to carry out the transfections. Passive lysis buffer (Promega) was used to prepare the cell lysates 48 h after transfection, and the Dual-Luciferase Reporter Assay Kit (Promega) was used to measure luciferase activities.
Cell proliferation and apoptosis assay.
CCK-8 (DOJINDO) assay was performed to measure the cellular proliferation rate of hepatocarcinoma cells. HepG2 cells were subjected to apoptosis assay. Forty-eight hours after transfection with miR-210 mimic/scramble, the cells were subjected to apoptosis analysis using the Annexin V-FITC Apoptosis Detection Kit I (BD Biosciences) according to the manufacturer by the FACS Calibur Flow Cytometer (Becton Dickinson).
Western blot
HepG2 and PLC cells were lysed using M-PER (Pierce). Supernatants were collected by centrifugation. Proteins were resolved on Tris-HCl gradient gel (Bio-Rad) and transferred to nitrocellulose membrane. After room temperature blocking (5% BSA) for 1 hour, the membranes were incubated overnight at 4 °C with antibodies for EFNA3 (Cell Signaling Technology), and GAPDH (Abcam) according to the manufacturer. After incubated with HRP-conjugated secondary antibodies from Santa Cruz (CA, USA) for 2 hours, the membranes were visualized using the ECL-Plus kit (Amersham Biosciences).
Statistical analysis
Statistical analyses were performed using SPSS 16.0 software (SPSS Inc., Chicago, IL, USA). Student’s t-test (two-tailed) was performed to compare 2 groups unless otherwise indicated (χ2 test), and one-way analysis of variance was performed to analyze 3 groups’ data. Correlations between miRNA expression and clinicopathological features were analyzed, non-parametric tests such as the Mann-Whitney U-test and the Kruskal-Wallis test were performed for differences between 2 groups and between 3 or more groups respectively. Each experiment was repeated at least 3 times. P values <0.05 were considered statistically significant.
Results
miR-210 is a prognostic factor of HCC chemotherapy
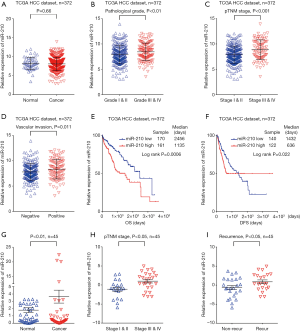
To elucidate the role of miR-210 in HCC chemotherapy, we first determined the correlation between miR-210 expression and HCC progression using the entire TCGA HCC cohort (372 patients). Although no significant difference in the expression of miR-210 was observed between the HCC tissues and the matched normal controls (Figure 1A), we noticed that miR-210 upregulation was significantly associated with a more advanced tumor phenotype (P<0.001) (Figure 1B,C) and a more extensive vascular invasion (P=0.011) (Figure 1D). Kaplan-Meier survival analysis demonstrated that lower miR-210 levels in patients were correlated with longer overall survival (OS) and disease-free survival (DFS) (Figure 1E,F). We also performed qRT-PCR analysis to determine the expression of miR-210 in a total of 45 pairs of fresh HCC tissue samples and adjacent non-cancer tissues. The results showed that miR-210 expression was significantly upregulated in HCC tissues compared with the matched adjacent non-cancer liver tissues (here denoted as normal control) (Figure 1G). The correlation between miR-210 expression and the TNM stage of HCC patients was further explored, we found that miR-210 upregulation was associated with advanced tumor stage (P<0.05) (Figure 1H). Notably, we explored whether better chemotherapy clinical potency was associated with lower miR-210 levels. Follow-up study was further performed within 45 cases of HCC patients underwent chemotherapy treatment after resection, and we divided these patients into 2 groups according to recurrence rate 3 years after resection. We found that HCC patients underwent recurrence had a higher miRNA-210 expression in the tumors (Figure 1I). These results indicate that miR-210 is involved in HCC development and may serve as a prognostic biomarker for HCC patients receiving chemotherapy.
miR-210 inhibition contributes to improved chemosensitivity of cisplatin in HCC cells
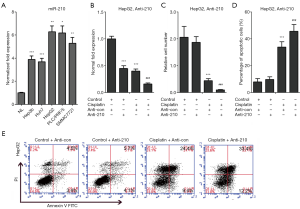
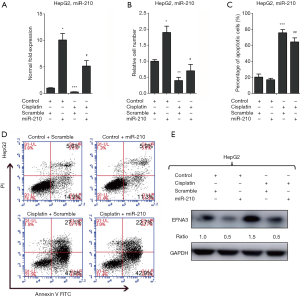
As mentioned above, HCC patients with low levels of miR-210 in their tumors represented a high response rate to cisplatin chemotherapy. Therefore, we further performed experiments to validate this finding in HCC cells (Figure 2). We knocked down the expression of miR-210 in HepG2 cells by transfection with a specific inhibitor of miR-210 (Anti-210) (Figure 2B). And then these cells were treated with cisplatin after the IC50 value was determined (34.9 mg/L). The following cell growth analysis revealed that miR-210 inhibition led to decreased cell proliferation in HepG2 cells (Figure 2C). We further performed apoptosis assay and observed that miR-210 inhibition promoted cisplatin-induced apoptosis in HepG2 cells (Figure 2D,E). Together, these data indicate that miR-210 suppression contributes to the improved efficacy of cisplatin in HCC cells.
EFNA3 is a direct target of miR-210 in HCC cells
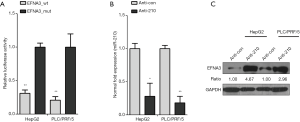
The best way to elucidate the function of miR-210 is to identify its direct targets. EFNA3 was validated as a miR-210 effector in peripheral nerve sheath tumor cells (22). In this study, we further confirmed this finding in HCC cells. We performed dual-luciferase reporter assay and observed that endogenous miR-210 inhibited the luciferase activity of wild-type EFNA3 3'-UTR when compared with mutant EFNA3 3'-UTR in HepG2 and PLC/PRF/5 cells (Figure 3A). Because miR-210 expression was significantly upregulation in advanced HCC tissues and cell lines, we next knocked down the expression of miR-210 in HCC cells to investigate its effect on the expression of EFNA3. HepG2 and PLC/PRF/5 cells were transfected with a miR-210 inhibitor, and qRT-PCR analysis revealed that miR-210 expression was decreased upon Anti-210 treatment (Figure 3B). Remarkedly, our western blot analysis indicated EFNA3 protein level was upregulated by miR-210 inhibition in HepG2 and PLC/PRF/5 cells (Figure 3C). Altogether, these data indicate EFNA3 is the target of miR-210 in HCC cells.
Cisplatin exhibits clinical efficacy through miR-210-induced EFNA3 signaling in HCC cells
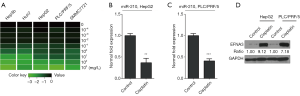
We next investigated whether cisplatin held efficacious effects on HCC treatment through miR-210 induced signaling. Firstly, the expression of miR-210 was determined in several HCC cell lines, including Hep3b, Huh7, HepG2, PLC/PRF/5, and SMMC7721. The results revealed that miR-210 expression was increased in these cells compared with primary human hepatocytes (NL) (Figure 2A). Next, the above cells were treated with cisplatin, and qRT-PCR analysis showed that miR-210 expression was downregulated by cisplatin in a dose-dependent manner (Figure 4A). The IC50 values in HepG2 and PLC/PRF/5 cells were estimated, and then we treated these two cells with cisplatin (HepG2, 34.9 mg/L; PLC, 21.1 mg/L). qRT-PCR analysis showed that miR-210 expression was significantly downregulated in HepG2 and PLC/PRF/5 cells upon cisplatin treatment (Figure 4B,C). Because cisplatin treatment led to a decrease of miR-210 levels and EFNA3 is a direct functional target of miR-210 in HCC cells as stated above, EFNA3 protein levels might be increased in HCC cells with cisplatin treatment. Western blot analysis confirmed this hypothesis (Figure 4D). Altogether, these findings indicate that miR-210-mediated EFNA3 pathway is a potential chemotherapeutic target of cisplatin in HCC cells.
miR-210 overexpression prevents the suppression of cisplatin treatment on HCC cell growth
Given the relationship between miR-210 and cisplatin efficacy, we further performed a rescue assay to elucidate the role of miR-210 in HCC chemotherapy with cisplatin. To this end, miR-210 mimic was transfected into HepG2 cells following cisplatin treatment to re-modify the levels of miR-210 and its target. qRT-PCR analysis showed that cisplatin treatment led to a decrease in miR-210 expression and this downregulation was partially rescued by miR-210 overexpression in HepG2 cells (Figure 5A). In addition, the cell proliferation assay demonstrated that miR-210 overexpression repressed cisplatin-induced proliferation arrest of HepG2 cells (Figure 5B). We also performed apoptosis analysis in HepG2 cells and observed that miR-210 overexpression repressed apoptosis induced by cisplatin (Figure 5C,D). We also observed that EFNA3 protein was increased after cisplatin treatment and decreased with miR-210 overexpression (Figure 5E). In summary, these findings suggest that cisplatin holds clinical benefits for HCC therapy at least partly through regulating miR-210-induced EFNA3 signaling.
Discussion
In the present work, we identified miR-210 as a miRNA involved in chemosensitivity of HCC. miR-210 was overexpressed in human advanced HCC and miR210 overexpression predicts higher disease stage, poor overall and disease-free survival as well as high incidence of recurrence after chemotherapy. We found that miR-210 could be inhibited by the drug cisplatin, and miR-210-mediated the effects of cisplatin by targeting EFNA3.
HCC is a kind of life-threatening solid cancer and chemotherapy is a critical therapeutic strategy for this disease. Cisplatin is a clinical drug for treatment of human HCC (23). However, the mechanisms underlying the cisplatin-mediated inhibition of HCC and the chemoresistance are not fully understood.
Increasing evidence clearly indicated that deregulation of miRNAs was involved in tumorigenesis and progression. In this pathological process, specific miRNAs function as oncomiRs or tumor suppressive miRNAs via their direct targets. Recent studies further demonstrated that miRNA expression signature served as prognostic biomarkers for cancer patients (24-26). However, the role of specific miRNAs, such as miR-210, in the chemotherapy or chemoresistance was not defined. Our data showed that miR-210 was a potential biomarker to predict the prognosis of HCC patients underwent chemotherapy according to the finding that HCC patients with high miR-210 levels in their tumors tended to recur.
We further observed that the expression of miR-210 was inhibited in HCC cells with cisplatin treatment, suggesting miR-210-induced signaling might be a potential target of cisplatin in HCC. We further confirmed this data by determining a direct bona fide effector of miR-210 in cisplatin-treated HCC cells, and we noticed that cisplatin-induced increased EFNA3 protein levels via miR-210. A rescue assay was conducted to clearly demonstrate the role of miR-210 in cisplatin exhibiting its suppressive impact on HCC cell growth, which revealed that miR-210 overexpression led to a significant alteration of cisplatin-induced cell growth arrest. These findings implicated that the miR-210-EFNA3 signaling is critically involved in the anti-tumor effect of cisplatin in HCC cells. Further in vivo growth or PDX experiments will be important to provide a full profile of miR-210-EFNA3 signaling in cisplatin efficiency in human HCC.
Conclusions
Our data provide the evidence that miR-210 is an effective biomarker for predicting the efficacy of HCC patients receiving chemotherapy. Targeting miR-210-induced signaling might be a novel strategy to improve the prognosis of HCC patients treated with cisplatin.
Table S1
| Characteristics | n [%] |
|---|---|
| Age, years | |
| <60 | 160 [45] |
| ≥60 | 195 [55] |
| Gender | |
| Male | 243 [68] |
| Female | 113 [32] |
| TNM stage | |
| Stage I & II | 247 [74] |
| Stage III & IV | 87 [26] |
| Tumor grade | |
| Grade 1–2 | 221 [63] |
| Grade 3–4 | 131 [37] |
| Vascular invasion | |
| None | 196 [65] |
| Micro | 91 [30] |
| Macro | 15 [5] |
HCC, hepatocellular carcinoma.
Table S2
| Target ID | Sequence |
|---|---|
| U6 snRNA RT | 5'-AAAATATGGAACGCTTCACGAATTTG-3' |
| U6 snRNA | |
| Forward | 5'-CTCGCTTCGGCAGCACATATACT-3' |
| Reverse | 5'-ACGCTTCACGAATTTGCGTGTC-3' |
| miR-210-3p RT | 5'-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTCAGCCG-3' |
| miR-210-3p miRNA | |
| Forward | 5'-CTACAACTGTGCGTGTGACAG-3' |
| Reverse | 5'-GTGCAGGGTCCGAGGT-3' |
| EFNA3 3'-UTR WT | |
| Forward | 5'-TCTAGACCCGGGCGGCCTTGTG-3' |
| Reverse | 5'-GCGGCCGCGGAGGATAGGTGGG-3' |
EFNA3, ephrin A3.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.03.26). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was previously approved by the ethical board of the Affiliated Tumor Hospital of Harbin Medical University (EAEC 2015-22) and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893-917. [Crossref] [PubMed]
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- Takizawa D, Kakizaki S, Sohara N, et al. Hepatocellular carcinoma with portal vein tumor thrombosis: clinical characteristics, prognosis, and patient survival analysis. Dig Dis Sci 2007;52:3290-5. [Crossref] [PubMed]
- Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology 2003;37:429-42. [Crossref] [PubMed]
- Reed ML, Vaitkevicius VK, Al-Sarraf M, et al. The practicality of chronic hepatic artery infusion therapy of primary and metastatic hepatic malignancies: ten-year results of 124 patients in a prospective protocol. Cancer 1981;47:402-9. [Crossref] [PubMed]
- Ambros V. The functions of animal microRNAs. Nature 2004;431:350-5. [Crossref] [PubMed]
- Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993;75:843-54. [Crossref] [PubMed]
- Lu J, Getz G, Miska EA, et al. MicroRNA expression profiles classify human cancers. Nature 2005;435:834-8. [Crossref] [PubMed]
- Iorio MV, Ferracin M, Liu CG, et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res 2005;65:7065-70. [Crossref] [PubMed]
- Iorio MV, Visone R, Di Leva G, et al. MicroRNA signatures in human ovarian cancer. Cancer Res 2007;67:8699-707. [Crossref] [PubMed]
- Ma L, Teruya-Feldstein J, Weinberg RA. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature 2007;449:682-8. [Crossref] [PubMed]
- Hou J, Lin L, Zhou W, et al. Identification of miRNomes in human liver and hepatocellular carcinoma reveals miR-199a/b-3p as therapeutic target for hepatocellular carcinoma. Cancer Cell 2011;19:232-43. [Crossref] [PubMed]
- Coppola N, de Stefano G, Panella M, et al. Lowered expression of microRNA-125a-5p in human hepatocellular carcinoma and up-regulation of its oncogenic targets sirtuin-7, matrix metalloproteinase-11, and c-Raf. Oncotarget 2017;8:25289-99. [Crossref] [PubMed]
- He X, Li J, Guo W, et al. Targeting the microRNA-21/AP1 axis by 5-fluorouracil and pirarubicin in human hepatocellular carcinoma. Oncotarget 2015;6:2302-14. [Crossref] [PubMed]
- Wang Y, Liu C, Luo M, et al. Chemotherapy-Induced miRNA-29c/Catenin-delta Signaling Suppresses Metastasis in Gastric Cancer. Cancer Res 2015;75:1332-44. [Crossref] [PubMed]
- Liang WC, Wang Y, Wan DC, et al. Characterization of miR-210 in 3T3-L1 adipogenesis. J Cell Biochem 2013;114:2699-707. [Crossref] [PubMed]
- Liu C, Zhou Y, Zhang Z. MiR-210: an important player in the pathogenesis of preeclampsia? J Cell Mol Med 2012;16:943-4. [Crossref] [PubMed]
- Qin Q, Furong W, Baosheng L. Multiple functions of hypoxia-regulated miR-210 in cancer. J Exp Clin Cancer Res 2014;33:50. [Crossref] [PubMed]
- Qiu J, Zhou XY, Zhou XG, et al. MicroRNA-210 knockdown contributes to apoptosis caused by oxygen glucose deprivation in PC12 cells. Mol Med Rep 2015;11:719-23. [Crossref] [PubMed]
- Song G, Jia H, Xu H, et al. Studying the association of microRNA-210 level with chronic hepatitis B progression. J Viral Hepat 2014;21:272-80. [Crossref] [PubMed]
- Yu F, Yang J, Ouyang J, et al. Serum microRNA-210 levels in different groups of chronic hepatitis B patients. Clin Chim Acta 2015;450:203-9. [Crossref] [PubMed]
- Wang Z, Yin B, Wang B, et al. MicroRNA-210 promotes proliferation and invasion of peripheral nerve sheath tumor cells targeting EFNA3. Oncol Res 2013;21:145-54. [Crossref] [PubMed]
- Brower V. Sorafenib plus cisplatin for hepatocellular carcinoma. Lancet Oncol 2016;17:e424. [Crossref] [PubMed]
- Dotan ZA, Fridman E, Spector Y, et al. MicroRNAs as prognostic markers for survival in renal cell carcinoma conventional type T 2-4. J Clin Oncol 2011;29:e21115. [Crossref]
- Schultz NA, Andersen KK, Roslind A, et al. Prognostic microRNAs in patients operated for pancreatic cancer. J Clin Oncol 2011;29:4061. [Crossref]
- Jiang J, Wu C, Zheng X, et al. Prognostic values of microRNAs in phase III clinical trial gastric cancer patients treated with S-1/oxaliplatin or doxifluridine/oxaliplatin. J Clin Oncol 2011;29:4073. [Crossref]


