
Clinicopathological and prognostic significance of NCALD protein expression in lung adenocarcinoma
Introduction
Lung cancer remains the leading cause of cancer-related death globally (1). In spite of tremendous efforts to improve the standard therapeutics, the prognosis of lung cancer is poor, with a 5-year survival rate as low as 15% (2,3). Among lung cancer, non-small cell lung cancer (NSCLC) is account for 85% of all cases and can be further categorized into two common subtypes, lung squamous cell carcinoma (LUSC) and lung adenocarcinoma (LUAD) (4,5). LUAD could be further classified into lepidic adenocarcinoma, acinar adenocarcinoma, papillary adenocarcinoma, micropapillary adenocarcinoma, solid adenocarcinoma and so on. Most patients with LUAD, the first time going to a doctor is usually started after cough or bloody sputum, which means most patients are diagnosed with LUAD in the advanced stage of the disease. The standard care of treatments for advanced stage LUAD patients are platinum-based doublet regimens and targeted cancer therapy. However, drug resistance and subsequent tumor progress are unavoidable. Therefore, searching novel diagnostic and prognostic marker of LUAD is essential for improving early diagnostic rate and patient outcomes.
NCALD, highly conserved across species, is a member of the visinin-like subfamily of EF hand calcium-binding proteins (6). It is reported that NCALD is abundant in axonal growth cones, cerebral neurons, spinal MNs and primarily involved in neuronal Ca2+ signaling (7). In fact, through a calcium dependent manner, NCALD could both interact with clathrin and regulates the activity of clathrin, which plays an important role in the process of endocytosis (8,9). Subsequent studies indicated that NCALD might be implicated in the pathogenesis of human cancer. A study conducted by Couvelard and colleagues documented that NCALD gene expression to be one of many genes which can distinguish between non-metastatic and metastatic pancreatic endocrine tumor tissues (10). What’s more, Isaksson et al. presented a whole transcriptome profile in whole blood cell mRNA of ovarian cancer patients by the Affymetrix Human Gene 1.0 ST Array (11). They found that the expression level of NCALD mRNA was decreased in the group with poorly differentiated, having advanced stage and poor prognosis tumors. Besides, other research also has shown that down-regulation of NCALD in asbestos-related lung cancers (12). Together, these findings are implied NCALD a novel biomarker specific to these cancer patients. Indeed, in the study conducted by Shi et al. (13), they have showed that NCALD protein was declined in NSCLC samples and high NCALD protein expression was significantly associated with increased survival overall.
Nonetheless, the relationship between the expression level of NCALD and more detailed clinicopathological feature of LUAD remains unknown. In this study, we sought to determine the clinical relevance of NCALD protein expression in 90 primary operable lung adenocarcinoma cases. We investigated the association between NCALD expression level and clinicopathological characteristics as well as OS were investigated.
Methods
Patient and tissue specimen
Data from 90 patients who underwent surgical resection for lung adenocarcinoma at Huzhou Hospital, Zhejiang University School of Medicine between July 2004 and June 2009 were retrospectively analyzed. Patients with other types of malignancy or received local or systemic treatment before any operation were excluded from this study. All pathologic specimens were independently reviewed by at 2 pathologists. The clinicopathologic information of the patients with lung adenocarcinoma, including age, sex, tumor size, tumor differentiation, lymph node metastasis and TNM (AJCC 7th) were recorded and summarized in Table 1. Survival data were obtained from patients’ medical records. The study protocol was approved by the Institutional Review Board of Huzhou Hospital and all of the participants signed an informed consent form.
Table 1
| Protein | Histotype | Expression levels (number) | P | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | |||
| NCALD | N | 1 | 1 | 17 | 39 | 18 | 12 | 2 | <0.01 |
| T | 30 | 8 | 25 | 19 | 7 | 1 | 0 | ||
N, normal tissue; T, tumor tissue; NCALD, neurocalcin delta; LUAD, lung adenocarcinoma.
Construction of tissue microarrays (TMAs)
Ninety cases of LUAD tissues and paired adjacent normal tissues were confirmed by reviewing hematoxylin and eosin (H&E) stained slides. One representative formalin-fixed paraffin-embedded archival block were selected for each case and used in the construction of tissues microarray. Using the TM-1 tissue microarray kit (Changzhou Ruipin Precision Instrument Co., Ltd., China), 2-µm thick tissue cores were extracted from individual paraffin blocks (donor blocks). They were subsequently re-arranged into recipient paraffin blocks (TMA blocks). The TMA blocks were incubated at 60 °C for about 30 min and cooled at normal temperature.
Immunohistochemistry (IHC)
Four µm thick tissue sections were obtained from TMA blocks and used for IHC. These sections were deparaffinized in xylene for 20 min and then re-hydrated in a graded series of decreasing alcohol series. For staining with anti-NCALD antibodies, antigen retrieval was performed in a pressure cooker with an epitope retrieval solution (pH 6) for 20 min, followed by washing of the specimens in phosphate buffered saline (PBS). Endogenous peroxidase activity was blocked with 0.3% H2O2 in methanol at 37 °C for 30 min. Tissues were then stained with IHC using a primary antibody in a humidified chamber at 4 °C overnight. The primary antibodies were a polyclonal antibody against NCALD (1:50 dilution; Proteintech, Chicago, IL, USA). Subsequently, a secondary antibody was then applied for 30 min and the sections then were treated with Vectastain ABC reagent for 30 min. All the slices were visualized with the DAB chromogen for 10 min and counterstained with hematoxylin/eosin. For negative control the primary antibody was omitted. Every step was followed by washing in PBS.
Immunohistochemical assessments
To quantify NCALD protein expression, IHC staining was separately evaluated by 2 pathologists. Both the staining intensity and staining extensity were evaluated and scored in randomly selected five representative fields of vision. The percentage of positive cells was scored as 0 (≤9% positive), 1 (10–25% positive), 2 (26–50% positive), 3 (51–100% positive). The staining intensity (0= no staining, 1= mild staining, 2= moderate staining and 3= strong staining). The final semi-quantitative IHC scores were calculated by adding up the strongest intensity score and the total extensity score. Immunostaining results were considered as follows: low expression level of NCALD when the score was 0–2, high expression level of NCALD when the score was 3–6.
Statistical analysis
All statistical analyses were conducted using SPSS (Statistical Package for the Social Sciences) software 19.0 (IBM, Chicago, IL, USA) and differences were considered to be statistically significant at P<0.05. Using Pearson’s χ2 and the t-tests, associations between NCALD expression level and patient clinicopathological parameters were assessed. Overall survival (OS) was defined as the time from the date of histological diagnosis to the date of last contact or death from any cause. The correlation of different survival time with LUAD characteristics, clinical features and NCALD were evaluated by using the Kaplan-Meier method. Univariate or multivariate Cox regression analysis were performed to assess whether a factor was an independent predictor of prognosis of LUAD.
Results
Expression of NCALD in LUAD as determined by immunohistochemistry
We first examined the expression level of NCALD protein in LUAD specimens and paired adjacent normal tissue using a large TMA. The tissue staining was semi-quantitatively scored by staining intensity and staining extensity, which have already been introduced in Materials and Methods. Via immunohistochemistry, we found that NCALD is mainly located in alveolar epithelium derived cells cytoplasm and LUAD had a significantly lower expression of NCALD expression in tumor tissue than in normal tissue (P<0.01, Figures 1 and 2A). NCALD protein expression was detected in 89/90 normal tissue, including low expression levels in 19 tissue (21%) and high expression levels in 71 tissue (79%). Nonetheless, NCALD protein was weakly or not expressed in LUAD tissue (low expression 70% and high expression 30%, Table 1). Images of representative immunostaining are presented in Figure 1.
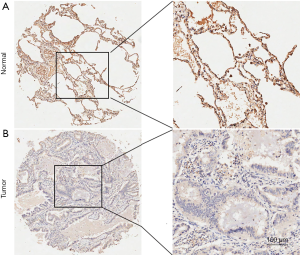
Relation between NCALD expression and clinicopathologic features
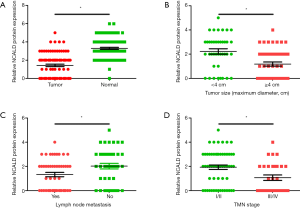
The clinicopathologic features of patients and the correlation with NCALD expression was analyzed in Table 2. In total, of the 90 patients with LUAD included in this study, 49 were men and 41 were women. The mean age at diagnosis was 63.5 years. Moreover, 40 patients with small tumor size (maximum diameter <4 cm) and 50 patients with large tumor size (maximum diameter ≥4 cm). Seventy cases had well or moderate differentiated tumor, 20 poorly differentiated. At the time of diagnosis, there were 49 patients showing signs of lymph node metastasis. Statistical analysis indicated that larger tumor diameter, which represent a higher tumor burden (Figure 2B), lymph node metastasis (Figure 2C), or more advanced tumors (Figure 2D) had lower NCALD protein expression. While, there was no significant association between NCALD expression and other clinical characteristics, such as differentiation, primary location or gender (P>0.05; Table 2).
Table 2
| Variables | N of cases (%) | NCALD protein expression level | ||
|---|---|---|---|---|
| Low (n=63) | High (n=27) | P | ||
| Age (year, mean =63.5) | 0.49 | |||
| <63.5 | 45 (50.0) | 30 | 15 | |
| ≥63.5 | 45 (50.0) | 33 | 12 | |
| Gender | 0.746 | |||
| Men | 49 (54.4) | 35 | 14 | |
| Women | 41 (45.6) | 28 | 13 | |
| Differentiation | 0.268 | |||
| Well, moderate | 70 (77.8) | 47 | 23 | |
| Poor | 20 (22.2) | 16 | 4 | |
| Tumor size (maximum diameter cm, mean =4.12) | <0.001 | |||
| <4 cm | 40 (44.4) | 21 | 19 | |
| ≥4 cm | 50 (55.6) | 42 | 8 | |
| Primary location | 0.546 | |||
| Left lung | 39 (43.3) | 26 | 13 | |
| Right lung | 51 (56.7) | 37 | 14 | |
| Lymph node metastasis | 0.03 | |||
| Positive | 49 (54.4) | 39 | 10 | |
| Negative | 41 (45.6) | 24 | 17 | |
| TMN stage | 0.015 | |||
| I/II | 60 (66.7) | 37 | 23 | |
| III/IV | 30 (33.3) | 26 | 4 | |
NCALD, LUAD, neurocalcin delta; lung adenocarcinoma.
Correlation between the NCALD protein expression level and prognosis of patients with LUAD
The Kaplan-Meier survival analysis indicated that low expression levels of NCALD protein were associated with poor prognosis of patients with LUAD (P<0.01, Figure 3A). What’s more, both lymph node metastasis and clinical TNM stages were statistically related to the prognosis of patients with LAUD (P<0.01, Figure 3B,C). Whereas, there were no significant differences in tumor location, tumor size and sex (P>0.05, Figure 3D,E,F). What’s more, well differentiation is related the good prognosis of patients with LUAD (Figure 3G).
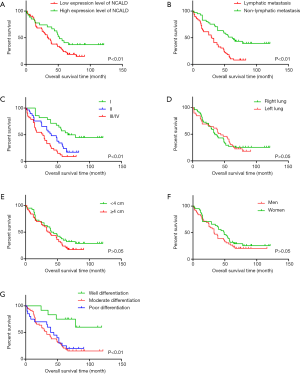
In the Cox regression model analysis, univariate analysis revealed that NCALD expression, lymph node metastasis, and clinical TNM stages were prognostic factors for patients’ survival (P<0.05, Table 3), which were consistent with the results of The Kaplan-Meier survival analysis. However, the multivariate Cox proportional hazards model demonstrated that there was no significant relation between NCALD expression and patients’ survival (Table 3).
Table 3
| Parameters | Overall survival | ||||
|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | ||||
| Exp (B) (95% CI) | P | Exp (B) (95% CI) | P | ||
| Gender (female vs. male) | 0.816 (0.507–1.313) | 0.403 | 1.143 (0.699–1.87) | 0.595 | |
| Age (<63.5 vs. ≥63.5) | 1.111 (0.693–1.781) | 0.663 | 0.889 (0.539–1.464) | 0.643 | |
| Differentiation (well/moderate vs. poor) | 1.093 (0.624–1.913) | 0.756 | 1.174 (0.65–2.12) | 0.596 | |
| Tumor size (<4 vs. ≥4 cm) | 1.363 (0.841–2.208) | 0.209 | 0.941 (0.537–1.651) | 0.833 | |
| Lymph node metastasis (absent vs. present) | 2.758 (1.660–4.581) | <0.001 | 0.496 (0.273–0.902) | 0.021 | |
| TMN stage (I/II vs. III/IV) | 2.501 (1.521–4.111) | <0.001 | 0.581 (0.32–1.056) | 0.075 | |
| NCALD expression (high vs. low) | 1.744 (1.004–3.029) | 0.048 | 1.23 (0.644–2.349) | 0.53 | |
CI, confidence interval; LUAD, lung adenocarcinoma; NCALD, neurocalcin delta.
Discussion
It is well known that genetic abnormalities contribute to lung tumorigenesis. In previous studies had shown that mutations in EGFR, inactivation of p53 and activation of K-RAS critical for the pathogenesis of lung cancer, especially lung adenocarcinoma (14-16). However, the prognosis of lung cancer is still poor. Aimed to improve early diagnostic rate and patient outcomes, we explored novel diagnostic and prognostic marker of lung adenocarcinoma in the present study. Here, we found that the expression level of NCALD protein was frequently low in human lung adenocarcinoma and that low expression of NCALD predicts the poor prognosis of advanced LUAD patients, which reveals that NCALD might be a prognostic biomarker for LUAD patients.
NCALD is a predominantly cytosolic neuronal calcium sensor binding protein (17). Several lines of evidence show that calcium plays a crucial role in miscellaneous cellular processes, such as differentiation, migration and proliferation of normal and neoplastic cells (18). NCALD belongs to visinin-like protein (VILIP) superfamily, which comprises NCALD, hippocalcin, VILIP1, VILIP2 and VILIP3 (19,20). Although the biological functions of VILIP superfamily members are largely unknown, continuing advances in cancer demonstrate that VILIP superfamily already attracted interest. It is reported that VILIP1 acts as a tumor suppressor gene and suppressed cell proliferation and invasiveness by decreasing MMP-9 and RhoA activity as well as reducing the expression of αV and α5 integrins in human squamous cell carcinoma cells (21,22). Subsequently, Fu et al. revealed that VILIP1 was downregulated in 11 aggressive NSCLC cell lines due to abnormal promoter hypermethylation and histone deacetylation (23). Furthermore, VILIP3, also named as HPCAL1, was found to inhibit HCC (hepatocellular carcinoma) cell proliferation by interacting with P21 directly resulted in increasing the stabilization of P21 in an ERK1/2-MAPK dependent manner (24). Importantly, in 2016, Shi and colleagues demonstrated that the expression of NCALD was repressed in NSCLC because of the combination of lncRNA 00673 and epigenetic repressor LSD1 (13). Taken together, these findings provide novel insight into the relationship between VILIP superfamily and human cancer.
Therefore, in the present study, we hypothesized that low expression of NCALD would be related to a poor prognosis in patients with LUAD. To verify the assumption, we use tissue microarrays. We discovered that NCALD protein was downregulated in LUAD and the low expression of NCALD significantly associated with tumor size, lymph node metastasis and TNM stage. In addition, we use both Kaplan-Meier survival analysis and Cox regression model analysis to investigate whether the expression of NCALD was associated with survival in LUAD patients. The result showed that there was a statistically significant correlation between survival and NCALD expression in Kaplan-Meier survival analysis and Cox regression univariate analysis. However, there was no statistically significant correlation between survival and tumor size in univariate and multivariate analysis. It might be caused by the cut-off value of tumor size, which is the median of tumor size. What’s more, an important shortage of this study was the lack of significant relationship between survival and TNM stage or NCALD expression in multivariate analyses due to the limitation of specimens. Hence, further studies of NCALD in LUAD cell lines will be needed to clarify its mechanisms after the preliminary results.
In summary, the expression of NCALD in patients with LUAD was found to be associated with a number of clinicopathologic factors, and importantly, was related to a good prognosis. If the functionality and molecular mechanisms of NCALD can be full identified, we might open avenues for the effect of NCALD on identification and treatment of LUAD.
Acknowledgments
Funding: The research was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.04.15). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the Institutional Review Board of Huzhou Hospital (No. 201805007), and all of the participants signed an informed consent form.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016; [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [Crossref] [PubMed]
- Cooper WA, Lam DC, O'Toole SA, et al. Molecular biology of lung cancer. J Thorac Dis 2013;5:S479-90. [PubMed]
- Ettinger DS, Akerley W, Bepler G, et al. Non-small cell lung cancer. J Natl Compr Canc Netw 2010;8:740-801. [Crossref] [PubMed]
- Wang W, Zhou Z, Zhao W, et al. Molecular cloning, mapping and characterization of the human neurocalcin delta gene (NCALD). Biochim Biophys Acta 2001;1518:162-7. [Crossref] [PubMed]
- Iino S, Kobayashi S, Hidaka H. Neurocalcin-immunopositive nerve terminals in the muscle spindle, Golgi tendon organ and motor endplate. Brain Res 1998;808:294-9. [Crossref] [PubMed]
- Riessland M, Kaczmarek A, Schneider S, et al. Neurocalcin Delta Suppression Protects against Spinal Muscular Atrophy in Humans and across Species by Restoring Impaired Endocytosis. Am J Hum Genet 2017;100:297-315. [Crossref] [PubMed]
- Ivings L, Pennington SR, Jenkins R, et al. Identification of Ca2+-dependent binding partners for the neuronal calcium sensor protein neurocalcin delta: interaction with actin, clathrin and tubulin. Biochem J 2002;363:599-608. [Crossref] [PubMed]
- Couvelard A, Hu J, Steers G, et al. Identification of potential therapeutic targets by gene-expression profiling in pancreatic endocrine tumors. Gastroenterology 2006;131:1597-610. [Crossref] [PubMed]
- Isaksson HS, Sorbe B, Nilsson TK. Whole genome expression profiling of blood cells in ovarian cancer patients -prognostic impact of the CYP1B1, MTSS1, NCALD, and NOP14. Oncotarget 2014;5:4040-9. [Crossref] [PubMed]
- Nymark P, Guled M, Borze I, et al. Integrative analysis of microRNA, mRNA and aCGH data reveals asbestos- and histology-related changes in lung cancer. Genes Chromosomes Cancer 2011;50:585-97. [Crossref] [PubMed]
- Shi X, Ma C, Zhu Q, et al. Upregulation of long intergenic noncoding RNA 00673 promotes tumor proliferation via LSD1 interaction and repression of NCALD in non-small-cell lung cancer. Oncotarget 2016;7:25558-75. [PubMed]
- Osada H, Takahashi T. Genetic alterations of multiple tumor suppressors and oncogenes in the carcinogenesis and progression of lung cancer. Oncogene 2002;21:7421-34. [Crossref] [PubMed]
- Belinsky SA. Silencing of genes by promoter hypermethylation: key event in rodent and human lung cancer. Carcinogenesis 2005;26:1481-7. [Crossref] [PubMed]
- Minna JD, Roth JA, Gazdar AF. Focus on lung cancer. Cancer Cell 2002;1:49-52. [Crossref] [PubMed]
- O'Callaghan DW, Ivings L, Weiss JL, et al. Differential use of myristoyl groups on neuronal calcium sensor proteins as a determinant of spatio-temporal aspects of Ca2+ signal transduction. J Biol Chem 2002;277:14227-37. [Crossref] [PubMed]
- Wickborn C, Klein-Szanto AJ, Schlag PM, et al. Correlation of visinin-like-protein-1 expression with clinicopathological features in squamous cell carcinoma of the esophagus. Mol Carcinog 2006;45:572-81. [Crossref] [PubMed]
- Burgoyne RD, Weiss JL. The neuronal calcium sensor family of Ca2+-binding proteins. Biochem J 2001;353:1-12. [Crossref] [PubMed]
- Jheng FF, Wang L, Lee L, et al. Functional contribution of Ca2+ and Mg2+ to the intermolecular interaction of visinin-like proteins. Protein J 2006;25:250-6. [Crossref] [PubMed]
- Mahloogi H, Gonzalez-Guerrico AM, Lopez De Cicco R, et al. Overexpression of the calcium sensor visinin-like protein-1 leads to a cAMP-mediated decrease of in vivo and in vitro growth and invasiveness of squamous cell carcinoma cells. Cancer Res 2003;63:4997-5004. [PubMed]
- Gonzalez Guerrico AM, Jaffer ZM, Page RE, et al. Visinin-like protein-1 is a potent inhibitor of cell adhesion and migration in squamous carcinoma cells. Oncogene 2005;24:2307-16. [Crossref] [PubMed]
- Fu J, Fong K, Bellacosa A, et al. VILIP-1 downregulation in non-small cell lung carcinomas: mechanisms and prediction of survival. PLoS One 2008;3:e1698. [Crossref] [PubMed]
- Zhang Y, Liu Y, Duan J, et al. Hippocalcin-like 1 suppresses hepatocellular carcinoma progression by promoting p21(Waf/Cip1) stabilization by activating the ERK1/2-MAPK pathway. Hepatology 2016;63:880-97. [Crossref] [PubMed]


