
Primary malignant mixed mullerian tumor of the peritoneum: a case report
Introduction
MMMT, also referred to as the malignant mixed mesodermal tumor and carcinosarcoma (1,2), is a highly aggressive biphasic neoplasm comprised coexisting elements of cancer and sarcoma. Mostly deriving from the genital tract in postmenopausal elderly women, such as the uterus or the ovary, it also can be found in neck and head, peritoneum, biliary tract and gastrointestinal tract (3). Primary peritoneal MMMT is extremely rare, and commonly occurs in the pelvic peritoneum. Furthermore, it has also been arisen in the serosal surface of the colon, rectal peritoneum, retroperitoneum, cul-de-sac, diaphragm peritoneum, anterolateral abdominal peritoneum, and omentum (4,5). Since the first reported by Ober and Black in 1955, only 41 cases have been described in the well documented literatures (6). In this paper, we present a peculiar case of primary peritoneal MMMT.
Case presentation
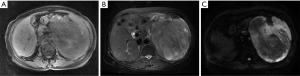
A 64-year-old woman was admitted to our department (First Affiliated Hospital of Zhejiang University, China) in April 2018 due to a discontinuous epigastric pain and an abdominal distention for 3 months. On physical examination, a palpable mass was touched in the left and upper quadrant. Initial complete blood count, liver, and kidney function tests were normal. Her serum CA 125 was 486.7 U/mL. The abdominal computed tomography (CT) scan demonstrated a 12×19 cm tumor with equal or low density, with multiple retroperitoneal enlarged lymph nodes, and the tumor’s margin in the artery phase obviously enhancing and delayed enhancing in the portal phase, uterus and adnexal region revealing no abnormality. The abdominal magnetic resonance imaging (MRI) revealed a giant tumor in the posterior peritoneum with iso-hypointense on T1WI, hyperintense on T2WI, uneven hyperintense or hypointense on diffusion weighted imaging (DWI), and progressive uneven enhancement in enhancement period (Figure 1). And then, the patient received an ultrasound-guided FNA of abdominal tumor in the left middle abdomen, and the initial pathological analysis indicated a highly malignant soft tissue sarcoma. According to pathological results and iconography’s characteristics, the patient was diagnosed as retroperitoneal sarcoma.
Treatment procedures
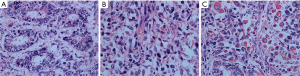
Retroperitoneal tumor resection with terminal segmental pancreatectomy and splenectomy were planned. On the operation, A 21 cm × 18 cm × 13 cm medium-texture tumor was found in the abdominal cavity, which has an undistinguishable boundary with the spleen. The tumor along with terminal segmental pancreas and spleen was resected. Histopathology of tumor showed biphasic differentiation, and epithelioid components with tumor cells of marked heteromorphism arranged in streak glands with structures. Stromal components were diffuse arrangement, infiltrative growth and spindle shaped with pleomorphic nuclei, rhabdomyosarcoma differentiation in some region, the pathological and immunohistochemical analysis suggestive of malignant mixed mullerian tumor (MMMT) with positive expression of CK7, CK, SMA, WT1, PAX-8, Ki-67 (75%) and negative expression of CD117, CD30, CK20, S-100, P53 (Figure 2). Postoperatively, the patient received three cycles chemotherapy of taxinol combined with carboplatin, the patient obtained partial response and the abdominal symptoms were obviously improved. Up to now, the patient has survived for 5 months.
Discussion
The terminology ‘mixed Müllerian tumor’ was made up of mesenchymal and epithelial elements of Müllerian origin. It generally classified into adenomyomas, adenofibromas, adenosarcomas, and carcinosarcomas (MMMT) according to the grade malignancy of the stromal and epithelial components (7). Tumors showing no differentiated sarcomatous elements have conventionally been known as homologous carcinosarcomas (8). Nevertheless, tumors consisted of carcinomatous elements in sarcomatous stroma with recognizable heterologous components, such as cartilage or muscles, went by the name of heterologous carcinosarcomas (8). The peritoneal carcinosarcoma’s pathogenesis has not been clarified at present. However, the most popular theory, known as the combination or conversion theory, is that both elements derived from a common, strange precursor or probably that the sarcomatous component composes of metaplastic transformation (9).
It is possible that carcinomatous and sarcomatous components are originated from an alike source, in view of the both components showing similar mutations (8). Rajanbabu et al. reported 36 cases with primary peritoneal MMMT, most of which originated in the pelvic peritoneum and uterine serosa, followed by the serous surface of the Colon, rectum, and other peritoneum (10). Consequently, it most often arises from genitourinary tract whereas could occur in any organ. MMMT, especially in peritoneum, is extremely rare with a poor prognosis and rapidly fatal course, regardless of tumor stage (11). El Hachem et al. (12) reported a case of the longest disease-free survival that was treated with a complete radical surgical treatment and adjuvant chemotherapy with 6 cycles of carboplatin and paclitaxel, and this patient was followed up to 113 months.
At present, the treatment of primary peritoneal MMMT is guided by experience of prior patients and treatment recommendations for uterine sarcoma, owing to limited data on its management and treatment. In terms of MMMT treatment, surgical tumor resection is the most effective method, and we should try to reach the status of no evidence of tumor by the surgeon’s unaided eye. Systemic adjuvant chemotherapy is essential for all patients after operation. Ifosfamide and cisplatin are usually the preferred chemotherapy regimen, and the reaction rate of ifosfamide is 25% and that of cisplatin as a single drug for uterine carcinosarcoma is 17% (13). The effect of radiotherapy is controversial for MMMT. There is no clear conclusion as to whether to undergo radiotherapy (11).
The patient reported by us underwent tumor resection firstly and received postoperative chemotherapy with 4 cycles of cisplatin combined with ifosfamide. Up to now, the patient has survived for 5 months and has been receiving current chemotherapy. Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.05.07). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nguyen GK, Berendt RC. Aspiration biopsy cytology of metastatic endometrial stromal sarcoma and extragenital mixed mesodermal tumor. Diagn Cytopathol 1986;2:256-60. [Crossref] [PubMed]
- Campins M, Madrenas J, Biosca M, et al. Extra-uterine müllerian carcinosarcoma. Acta Obstet Gynecol Scand 1986;65:811-2. [Crossref] [PubMed]
- Ambrosini-Spaltro A, Vaira V, Braidotti P, et al. Carcinosarcoma of the colon: report of a case with morphological, ultrastructural and molecular analysis. BMC Cancer 2006;6:185. [Crossref] [PubMed]
- Ohno S, Kuwano H, Mori M, et al. Malignant mixed mesodermal tumour of the peritoneum with a complete response to cyclophosphamide. Eur J Surg Oncol 1989;15:287-91. [PubMed]
- Garde JR, Jones MA, McAfee R, et al. Extragenital malignant mixed müllerian tumor: review of the literature. Gynecol Oncol 1991;43:186-90. [Crossref] [PubMed]
- Ober WB, Black MB. Neoplasms of the subcoelomic mesenchyme: report of two cases. AMA Arch Pathol 1955;59:698-705. [PubMed]
- D'Angelo E, Prat J. Pathology of mixed Müllerian tumours. Best Pract Res Clin Obstet Gynaecol 2011;25:705-18. [Crossref] [PubMed]
- Ko ML, Jeng CJ, Huang SH, et al. Primary peritoneal carcinosarcoma (malignant mixed mullerian tumor): Report of a case with five-year disease free survival after surgery and chemoradiation and a review of literature. Acta Oncol 2005;44:756-60. [Crossref] [PubMed]
- Cantrell LA, Havrilesky L, Moore DT, et al. A multi-institutional cohort study of adjuvant therapy in stage I-II uterine carcinosarcoma. Gynecol Oncol 2012;127:22-6. [Crossref] [PubMed]
- Rajanbabu A, Ahmad SZ, Vijaykumar DK, et al. The significance of the site of origin in primary peritoneal carcinosarcoma: case report and literature review. Ecancermedicalscience 2013;7:295. [PubMed]
- Serrano Diana C, Gómez García MT, López Del Cerro E, et al. Primary peritoneal carcinosarcoma. J Obstet Gynaecol 2017;37:398-9. [Crossref] [PubMed]
- El Hachem G, Jungels C, Larsimont D, et al. Retroperitoneal mixed malignant mullerian tumor: exceptional localisation and prognosis. Rev Med Brux 2018;39:146-9. [Crossref] [PubMed]
- Vaysse C, Touboul C, Filleron T, et al. Early stage (IA-IB) primary carcinoma of the fallopian tube: case-control comparison to adenocarcinoma of the ovary. J Gynecol Oncol 2011;22:9-17. [Crossref] [PubMed]


