The secondary surgery provides significant benefits to recurrent thymoma: a retrospective analysis based on Surveillance, Epidemiology and End Results database
Introduction
Surgery is the cornerstone of treatment for primary thymoma (1). The diagnostic age of patients, Masaoka stage, WHO pathologic classification, thymoma size, surgical treatment, and postoperative radiotherapy (PORT) are prognostic factors for thymoma (2-4). Besides, diagnostic age, thymoma size, clinical stage are predictive factors for tumor recurrence (5,6). Apart from these, appropriate adjuvant therapies could significantly prolong overall survival (OS) and progressive free survival (PFS) (2-4). These factors are identified regarding primary thymoma, however, rare researches focus on recurrent thymoma (RT).
Efficient therapies for RT include secondary surgery, adjuvant PORT, adjuvant chemotherapy, molecular targeted therapy, and immunotherapy. However, there is no effective therapeutic guideline for RT applied to clinical work (2,5). Some scholars suggested RT received secondary surgery had better survival than those who received conservative therapies (7-9). Whereas, others suggested secondary surgery could cause high morbidity and mortality (10). The efficacy of secondary surgery for RT after primary surgery remains controversial. Besides, complete studies of recurrent thymic tumors focused on thymoma and thymic carcinoma. Compared with thymoma, thymic carcinoma is an utterly different disease which has more aggressive oncologic appearance and higher possibility of distant metastasis, which probably leads confusions about the definition of resectable thymoma and selected criteria of secondary surgery (6).
In this work, data was reviewed from the Surveillance, Epidemiology, and End Results database (SEER). We discussed the indication of operation for RT, evaluated the efficacy of secondary surgery for RT, and provided evidences to prevent thymoma progression.
Methods
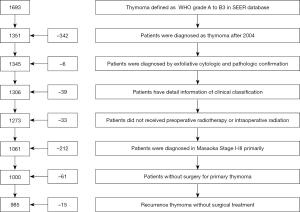
From January 1973 to December 2014, surgical treated patients diagnosed as thymoma, from type A to B3 by WHO pathologic classification, in SEER were reviewed. The detail selected process was listed in Figure 1. The exclusive criteria were: (I) the diagnostic year before 2004; (II) patients with oncologic history; (III) diagnostic methods without cytologic and pathologic confirmation; (IV) patients without detail clinical data or follow-up information; (V) patients with preoperative or intraoperative radiotherapy; (VI) primary thymoma with metastasis (Masaoka stage IV).
Patients’ parameters were abstracted including demographics, tumor size, pathological category, clinical classification (SEER summary clinical stage could be converted into Masaoka stage (11), therapeutic details, disease recurrence status (recurrence was confirmed by exfoliative cytology and/or pathology of secondary operative specimen), therapeutic regimens for recurrences, and outcomes. Patients were separated into without RT, second surgery for RT, and conservative treatments for RT 3 subgroups.
Statistical analysis was performed by SPSS software, version 23.0 (SPSS Inc., Chicago, USA). Continuous and categorical variables were compared by Student t-test and χ2 test respectively. OS was calculated by Kaplan-Meier method and Log-rank test. Independent predictors were identified by Cox proportional-hazards regression model. Survival time was calculated from the time of primary surgery to death or censored. A two-tailed P value <0.05 was considered statistically significant. Hazard ratio (HR) was presented with their 95% confidence interval (CI). Between without recurrence and surgical treated RT subgroups, 1:1 propensity score matching (PSM) was performed by using the caliper match algorithm with a width of 0.1 of the standard deviation of the logit for the propensity score to evaluate the efficacy of secondary surgery for resectable RT.
Results
Baseline of patients
A total of 1,000 patients were enrolled in this work (Figure 1). The median follow-up time was 61 months (range from 0–156 months) for all patients. As Table 1 showed that 185 (18.5%) patients were RT, the median diagnostic age of thymoma without recurrence after primary surgery was significantly younger than RT (57.2±14.1 vs. 67.3±12.1 years, P=0.006). The proportion of patients with PORT in RT was significantly lower than thymoma without recurrence after primary surgery (35.1% vs. 47.0%, P=0.003). The distribution of other factors between RT and thymoma without recurrence after primary surgery had no significant difference.
Table 1
| Variables | NRT (%) | RT (%) | P value |
|---|---|---|---|
| Age (years) | 57.2±14.1 | 67.3±12.1 | 0.006* |
| Gender | 0.471# | ||
| Male | 399 (49.0) | 96 (51.9) | |
| Female | 416 (51.0) | 89 (48.1) | |
| Tumor size (mm) | 70.0 | 61.5 | 0.159* |
| Masaoka stage | 0.380# | ||
| I–IIa | 350 (42.9) | 86 (46.5) | |
| IIb–III | 465 (57.1) | 99 (53.5) | |
| WHO grade | 0.308# | ||
| A–B1 | 451 (55.3) | 110 (59.5) | |
| B2–B3 | 364 (44.7) | 75 (40.5) | |
| PORT | 0.003# | ||
| Without | 432 (53.0) | 120 (64.9) | |
| With | 383 (47.0) | 65 (35.1) | |
| CHT | 0.315# | ||
| Without | 690 (84.7) | 162 (87.6) | |
| With | 125 (15.3) | 23 (12.4) | |
| Sum | 815 | 185 |
*, P value was calculated by Student t-test; #, P value was calculated by χ2 test. RT, recurrence thymoma; NRT, thymoma without recurrence; PORT, postoperative radiotherapy; CHT, chemotherapy.
OS for different therapies
According to the different therapeutic strategies, group without RT and RT had 815, 185 patients, respectively. In RT subgroup, 170 patients received secondary surgical treatment, and 15 patients received conservative treatments. As Table 2 showed that OS of conservative treated RT (5-year rate: 26.7%, time: 38.1 months) was significantly worse than patients without recurrence after primary surgery (5-year rate: 83.9%, time: 108.2 months) and secondary surgery treated RT patients (5-year rate: 78.2%, time: 101.3 months) (P<0.001, respectively). OS between patients without recurrence and secondary surgery treated RT had no significant difference (P=0.066). Secondary surgery for RT could significantly prevent disease progression.
Table 2
| Therapy | No. | OS | P value | |||||
|---|---|---|---|---|---|---|---|---|
| 5-year rate (%) | Time (Mo.) | 95% CI | NRT | R-S | R-C | |||
| NRT | 815 | 83.9 | 108.2 | 105.0–111.5 | 0.066 | <0.001 | ||
| R-S | 170 | 78.2 | 101.3 | 93.3–109.4 | 0.066 | <0.001 | ||
| R-C | 15 | 26.7 | 38.1 | 15.9–60.3 | <0.001 | <0.001 | ||
*, P value was calculated by Log-rank test. RT, recurrence thymoma; NRT, thymoma without recurrence; R-S, surgical treated RT; R-C, conservative treatments treated RT; Mo., months; CI, confidence interval.
Prognostic factors for thymoma patients
PSM was performed to match baseline factors to balance without RT patients and secondary surgical treated RT patients simultaneously: diagnostic age, gender, Masaoka stage, pathologic grade, adjuvant therapies. After PSM, the number of patients in each subgroup was 168 respectively. The distributed differences based on these factors were eliminated (Table 3). OS between without RT patients and secondary surgical treated RT patients had no significant difference (Figure 2). Besides, secondary surgery for RT could not shorten OS in multivariate analysis (Table 4). Thus, secondary surgical treated RT patients had comparable outcomes as patients without RT after primary surgery.
Table 3
| Variables | Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|---|
| NRT (%) | R-S (%) | P value | N-R (%) | R-S (%) | P value | ||
| Age (years) | 57.2±14.1 | 66.4±12.0 | 0.000* | 65.8±12.8 | 66.2±11.9 | 0.339* | |
| Gender | 0.699# | 0.662# | |||||
| Male | 399 (49.0) | 86 (50.6) | 88 (52.4) | 84 (50.0) | |||
| Female | 416 (51.0) | 84 (49.4) | 80 (47.6) | 84 (50.0) | |||
| Masaoka stage | 0.399# | 0.827# | |||||
| I–IIa | 350 (42.9) | 79 (46.5) | 76 (45.2) | 78 (46.4) | |||
| IIb–III | 465 (57.1) | 91 (53.5) | 92 (54.8) | 90 (53.6) | |||
| WHO grade | 0.163# | 0.911# | |||||
| A–B1 | 451 (55.3) | 104 (61.2) | 101 (60.1) | 102 (60.7) | |||
| B2–B3 | 364 (44.7) | 66 (38.8) | 67 (39.9) | 66 (39.3) | |||
| PORT | 0.037# | 0.578# | |||||
| Without | 432 (53.0) | 105 (61.8) | 98 (58.3) | 103 (61.3) | |||
| With | 383 (47.0) | 65 (38.2) | 70 (41.7) | 65 (38.7) | |||
| CHT | 0.162# | 0.469# | |||||
| Without | 690 (84.7) | 151 (88.8) | 153 (91.1) | 149 (88.7) | |||
| With | 125 (15.3) | 19 (11.2) | 15 (8.9) | 19 (11.3) | |||
| Sum | 815 | 170 | 168 | 168 | |||
*, P value was calculated by Student t-test; #, P value was calculated by χ2 test. RT, recurrence thymoma; NRT, thymoma without recurrence; R-S, surgical treated RT; PORT, postoperative radiotherapy; CHT, chemotherapy; PSM, propensity score matching.
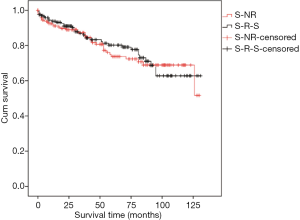
Table 4
| Variables | Overall survival | ||||||
|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | ||||||
| 5-year time (Mo.) | Rate (%) | P value | HR | 95% CI | P value | ||
| Age (year) | <0.001 | <0.001 | |||||
| ≤65 | 118.9 | 91.3 | 1.000 | ||||
| >65 | 88.6 | 68.8 | 4.399 | 2.396–8.077 | |||
| Gender | 0.146 | 0.210 | |||||
| Male | 106.4 | 81.4 | 1.000 | ||||
| Female | 97.0 | 76.2 | 1.356 | 0.843–2.183 | |||
| Masaoka stage | 0.012 | 0.018 | |||||
| I–IIa | 109.9 | 86.4 | 1.000 | ||||
| IIb–III | 95.7 | 72.5 | 1.928 | 1.118–3.326 | |||
| WHO grade | 0.004 | 0.003 | |||||
| A–B1 | 107.6 | 83.7 | 1.000 | ||||
| B2–B3 | 92.2 | 71.4 | 2.046 | 1.269–3.298 | |||
| Treatment | 0.782 | 0.565 | |||||
| NRT | 100.7 | 78.6 | 1.000 | ||||
| R-S | 102.5 | 79.2 | 0.871 | 0.544–1.394 | |||
| PORT | 0.395 | 0.044 | |||||
| Without | 100.7 | 79.6 | 1.000 | ||||
| With | 104.3 | 77.8 | 0.604 | 0.378–0.987 | |||
| CHT | 0.230 | 0.719 | |||||
| Without | 103.0 | 79.8 | 1.000 | ||||
| With | 93.0 | 70.6 | 1.136 | 0.567–2.274 | |||
RT, recurrence thymoma; NRT, thymoma without recurrence; R-S, surgical treated RT; PSM, propensity score matching; Mo., months; PORT, postoperative radiotherapy; CHT, chemotherapy; HR, hazard ratio; CI, confidence interval.
Besides, patients younger than 65 years had significantly better OS (5-year rate, 91.3% vs. 68.8%; time, 118.9 vs. 88.6 months; P<0.001). With the advancement of Masaoka stage (I–IIa vs. IIb–III: 5-year rate, 86.4% vs. 72.5%; time, 109.9 vs. 95.7 months; P=0.012) and pathologic grade (A–B1 vs. B2–B3: 5-year rate, 83.7% vs. 71.4%; time, 107.6 vs. 92.2 months; P=0.004), OS of patients decreased significantly. All these variables were adjusted by multivariate analysis. HR of diagnostic age (elder 65 vs. younger 65 years: HR, 4.399, 95% CI: 2.396–8.077, P<0.001), Masaoka stage (IIb–III vs. I–IIa: HR, 1.928, 95% CI: 1.118–3.326, P=0.018), pathologic grade (B2–B3 vs. A–B1: HR, 2.046, 95% CI: 1.269–3.298, P=0.003), and PORT (with vs. without: HR, 0.604, 95% CI: 0.378–0.987, P=0.044) for OS showed significant differences.
Discussion
The present work reviewed surgical treated thymoma patients from SEER to evaluate the safety and efficacy of secondary surgery for RT patients, and identify independent predictive factors for RT. The principle observation is secondary surgery provides comparable outcomes for RT to patients without recurrence after primary surgery.
Thymoma and thymic carcinoma are components of thymic tumors, but distinctly different diseases. Compared with thymic carcinoma, thymoma has indolent oncologic appearance, lower propensity of distant metastasis, and better outcome (11,12). The majority of previous studies did not separate these 2 kinds of thymic tumor probably causes confusions concerning secondary surgical selected criteria (6). Meanwhile, since the low incidence and recurrent rate of thymoma, studies focused on therapeutic regimens for RT are rare.
Previous studies investigated independent predictive factors of thymoma including age, Masaoka stage, WHO pathologic grade, resected marginal status, and adjuvant therapies (2,4,13-16). Besides, patients with myasthenia gravis could influence survival (17). Finally, RT with appropriate adjuvant therapies would have comparable outcomes as N-R (2,5,18).
Previous study confirmed distant metastasis accounts for extremely small proportion in RT, which needs better systemic therapies (19). The majority of RT occurs as intrathoracic disease which has opportunity to receive secondary surgery (5). Whereas, survival is a critical parameter to evaluate the therapeutic safety and efficacy. Some scholars suggested that OS, 3-, 5-, and 10-year survival rate of R-S were significantly better than R-C (6-8,20). On the other hand, others suggest that secondary surgery should be considered in strictly selected patients because secondary surgery could not provide benefits for RT with invasiveness of the lesion and lead high mortality (9,20). In the clinic, the rate of secondary surgery treated RT is relatively high (5,6). Herein, the selected criteria of resectable RT should be estimated, the efficacy of secondary surgery should be discussed.
In this work, R-S has significantly better outcomes than R-C. Besides, OS has no significant discrepancy between R-S and N-R. According to observations, secondary surgery for RT could be advocated if patients could tolerate surgery and tumor could be removed completely. However, disease free survival (DFS) of re-surgery patients could not be reviewed in database, which is a limitation to evaluate the efficacy of secondary surgery for RT.
The definition of resectable thymoma remains controversial, therefore, the efficacy of re-surgery and appropriate re-surgical type should be discussed. In present work, in order to avoid the bias caused by parameters such as oncologic malignancy and progression between RT and N-R, PSM was performed. After PSM, the demographics, oncologic parameters, and adjuvant therapies between RT and N-R have no significant difference. We believe that the only difference between the 2 groups is postoperative therapies. Interestingly, OS of patients between N-R and R-S has no significant difference. It is suggested that appropriate re-surgery provides significant benefits for Masaoka stage I-III RT. It is easy to understand that patient with an easily resectable intrathoracic recurrence would be performed secondary surgery (6). Besides, some scholars suggested that surgical treated thymoma with visceral, parietal pleura, pericardial, or epicardial surfaces recurrence determines the type of re-surgery, including pleuropneumonectomy, radical pleurectomy/decortication, and local excision of pleural, pericardial, and diaphragmatic implants. Appropriate surgical regimens could provide long-term survival of Masaoka stage IVa RT (20,21). Therefore, Masaoka stage I–IVa RT would have an opportunity to undergo secondary surgery.
Secondary surgery could prolong survival provides powerfully therapeutic evidences for resectable RT. However, selected criteria and the appropriate regimens of secondary surgery for RT in different types should be investigated in the further study.
Limitation
As a large population retrospective database, SEER has its limitations inevitably. It does not include the details of recurrence (16). Fortunately, as an indolent tumor, the distant recurrence rate of thymoma is extremely low. On the other hand, the overwhelming majority of RT occurs in mediastinum or thoracic cavity, which provides opportunity to perform secondary surgery. Secondly, the information concerning adjuvant chemotherapeutic regimen, dosage, toxicity, side-effects is not enrolled in SEER (16). Although, these factors are not focuses in the present work, based on strict statistical calculation, the efficacy of the adjuvant therapies and the discrapancy of survival based on different therapies are clear, reasonable, and reliable. Thirdly, the relationship between secondary surgical approaches and survival could not be discussed because of the incomplete information. Fourthly, the information of resected scope and margins of primary and secondary surgeries is missing, which may hinder the discussion of surgical indications. The issues were caused by limitations which would be solved by using the database from our own single-center in the future.
Conclusions
Elder patients, advanced Masaoka stage, upgraded pathologic grade, and without PORT indicate significantly worse OS in thymoma. In addition, Masaoka stage I–III RT with secondary surgery could provide acceptable outcomes compared to thymoma patients without recurrence after primary surgery. Finally, selected criteria of secondary surgery for RT patients should be investigated in the further study.
Acknowledgments
Funding: This study was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.05.09). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This article does not contain studies involving human participants or the use of experimental animals by any of the authors.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology (NCCN Guidelines) Thymomas and Thymic Carcinomas. Version 2.2018, 02/16/18.
- Bian D, Zhou F, Yang W, et al. Thymoma size significantly affects the survival, metastasis and effectiveness of adjuvant therapies: a population based study. Oncotarget 2018;9:12273-83. [Crossref] [PubMed]
- Jackson MW, Palma DA, Camidge DR, et al. The Impact of Postoperative Radiotherapy for Thymoma and Thymic Carcinoma. J Thorac Oncol 2017;12:734-44. [Crossref] [PubMed]
- Okereke IC, Kesler KA, Morad MH, et al. Prognostic indicators after surgery for thymoma. Ann Thorac Surg 2010;89:1071-7; discussion 1077-9. [Crossref] [PubMed]
- Ströbel P, Bauer A, Puppe B, et al. Tumor recurrence and survival in patients treated for thymomas and thymic squamous cell carcinomas: a retrospective analysis. J Clin Oncol 2004;22:1501-9. [Crossref] [PubMed]
- Bott MJ, Wang H, Travis W, et al. Management and outcomes of relapse after treatment for thymoma and thymic carcinoma. Ann Thorac Surg 2011;92:1984-91; discussion 1991-2.
- Regnard JF, Zinzindohue F, Pierre MP, et al. Results of re-resection for recurrent thymomas. Ann Thorac Surg 1997;64:1593-8. [Crossref] [PubMed]
- Ruffini E, Mancuso M, Oliaro A, et al. Recurrence of thymoma: analysis of clinicopathologic features, treatment, and outcome. J Thorac Cardiovasc Surg 1997;113:55-63. [Crossref] [PubMed]
- Sandri A, Cusumano G, Lococo F, et al. Long-term results after treatment for recurrent thymoma: a multicenter analysis. J Thorac Oncol 2014;9:1796-804. [Crossref] [PubMed]
- Haniuda M, Kondo R, Numanami H, et al. Recurrence of thymoma: clinicopathological features, re-operation, and outcome. J Surg Oncol 2001;78:183-8. [Crossref] [PubMed]
- Zhao Y, Gu H, Fan L, et al. Comparison of clinical features and survival between thymic carcinoma and thymic carcinoid patients. Eur J Cardiothorac Surg 2017;52:33-8. [Crossref] [PubMed]
- Huang J, Rizk NP, Travis WD, et al. Comparison of patterns of relapse in thymic carcinoma and thymoma. J Thorac Cardiovasc Surg 2009;138:26-31. [Crossref] [PubMed]
- Maniscalco P, Tamburini N, Quarantotto F, et al. Long-term outcome for early stage thymoma: comparison between thoracoscopic and open approaches. Thorac Cardiovasc Surg 2015;63:201-5. [Crossref] [PubMed]
- Detterbeck FC, Zeeshan A. Thymoma: current diagnosis and treatment. Chin Med J (Engl) 2013;126:2186-91. [PubMed]
- Chen YD, Feng QF, Lu HZ, et al. Role of adjuvant radiotherapy for stage II thymoma after complete tumor resection. Int J Radiat Oncol Biol Phys 2010;78:1400-6. [Crossref] [PubMed]
- Bian D, Qi M, Hu J, et al. The comparison of predictive factors regarding prognoses and invasion of thymic neuroendocrine tumors preoperatively and postoperatively. J Thorac Dis 2018;10:1657-69. [Crossref] [PubMed]
- Chen G, Marx A, Chen WH, et al. New WHO histologic classification predicts prognosis of thymic epithelial tumors: a clinicopathologic study of 200 thymoma cases from China. Cancer 2002;95:420-9. [Crossref] [PubMed]
- Fernandes AT, Shinohara ET, Guo M, et al. The Role of Radiation Therapy in Malignant Thymoma A Surveillance, Epidemiology, and End Results Database Analysis. J Thorac Oncol 2010;5:1454-60. [Crossref] [PubMed]
- Mengoli MC, Longo L, Varini S, et al. Invasive Medullary Type A Thymoma With Recurrent Distant Metastases. Ann Thorac Surg 2017;103:e423-5. [Crossref] [PubMed]
- Kaba E, Ozkan B, Erus S, et al. Role of Surgery in the Treatment of Masaoka Stage IVa Thymoma. Ann Thorac Cardiovasc Surg 2018;24:6-12. [Crossref] [PubMed]
- Marulli G, Margaritora S, Lucchi M, et al. Surgical treatment of recurrent thymoma: is it worthwhile?†. Eur J Cardiothorac Surg 2016;49:327-32. [Crossref] [PubMed]