
Association between smoking and neutrophil to lymphocyte ratio among prostate cancer survivors: the National Health and Nutrition Examination Survey
Introduction
Prostate cancer is one of the leading causes of cancer death among men (1). As of 2015, there were over three million men living with prostate cancer in the United States (1). The number of prostate cancer diagnoses and deaths annually was 112.6 per 100,000 men and 19.5 per 100,000 men, respectively. Incidence rates for prostate cancer remain the third most common type of cancer, with an estimated 164,690 new cases and another 29,430 deaths in 2018 (1). According to 2019 cancer statistics, prostate cancer was the second leading cause of cancer deaths among males in the United States (2).
Cigarette smoking has been associated with an increased risk of prostate cancer mortality, cancer recurrence, and prognosis (3-8). Gong et al. conducted a population-based study and demonstrated smoking at the time of prostate cancer diagnosis was strongly associated with prostate cancer-specific mortality (3). A cohort study conducted by Murta-Nascimento et al. demonstrated that smoking is associated with higher rate of prostate cancer-specific mortality and all-cause mortality (4). Another study carried out by Polesel et al. examining the negative impact of cigarette smoking on survival post-prostate cancer diagnosis found that not only current smoking was negatively associated with prostate cancer specific death, but smokers at diagnosis presented increased risks of all-cause and prostate cancer death (5). In addition, systematic review studies have reported that smokers with prostate cancer have significantly worse overall survival, prostate cancer specific survival, and recurrence-free survival as compared to never smokers (6-8). Although the biological mechanism through which cigarette use effects prostate cancer progression and mortality remains unclear, systemic inflammation has been considered as a potential pathway by which the association between smoking and prostate cancer progression occurs (3-5).
Inflammatory response has been proposed as an important marker in tumor development and cancer progression (9,10). Neutrophil-to-lymphocyte ratio (NLR) is an inflammatory marker (11). The variety in average of NLR has been observed in different racial populations without cancer diagnosed. The average of NLR among non-Hispanic whites, non-Hispanic blacks, and other non-Hispanics is 2.24, 1.76, and 2.10, respectively (11). An elevated ratio of NLR is associated with an increase in the concentration of pro-inflammatory cytokines, ultimately contributing to cellular damage and the development of metastases (12,13). Shafique et al. examined the effect of NLR on the 5-year relative survival and relative excess risk of death. The study found that higher NLR was a significant predictor of mortality, suggesting that systemic inflammation markers predict prostate cancer-specific survival (14). Another study reported that NLR was an independent prognostic marker with potential for use as an individual risk assessment for patients with prostate cancer. This study also stated that increased NLR was identified as a prognostic factor for clinical progression-free survival (15). Moreover, three meta-analyses reported higher NLR as a strong predictor of poor prognosis, progression-free survival, recurrence-free survival, and overall survival in metastatic castration-resistant prostate cancer survivors (16-18). The findings from these research support that NLR could serve as an indicator of the efficacy of treatment for prostate cancer. In addition to prostate cancer, previous studies examining various cancers such as ovarian cancer (19), lung cancer (20), breast cancer (21), esophageal cancer (22), and hepatocellular carcinoma (23) indicate that elevated NLR is also a significant predictor of adverse outcomes.
The negative impact of cigarette smoking on NLR has been examined among healthy populations. Previous literature suggests that current smokers are more likely to have an elevated ratio of NLR as compared to non-smokers (24-26). However, the association between cigarette smoking and NLR in prostate cancer survivors has not been thoroughly explored. Therefore, this study aimed to investigate the association between smoking and NLR among prostate cancer survivors using the U.S. population-based National Health and Nutrition Examination Survey (NHANES) data.
Methods
Study population
This study applied a cross-sectional study design using NHANES data, conducted by the National Center for Health Statistics of Centers for Disease Control and Prevention (CDC). Combined data from six consecutive 2-year survey cycle data sets were examined (2005–2006, 2007–2008, 2009–2010, 2011–2012, 2013–2014, and 2015–2016). A total of 354 male adults aged ≥20 years old and with a valid response to the related questions in smoking and prostate cancer were included. NHANES is a United States nationally representative survey based on a multistage, stratified and probability cluster sampling design, which is conducted by the by the National Center for Health Statistics of CDC (27). After an eligible sample person is selected, a face to face interview is performed at participant’s home by trained research staffs. The interview collects person-level demographic, health, and nutrition information; as well as the information of examination such as physical measurements and dental examinations. In addition, participant’s blood and urine specimens are collected for laboratory testing after their approval (27). Survey protocol was approved by the National Center for Health Statistics Research Ethics Review Board (28). Written informed consent was signed by each participant before conducting the data collection. The present study was reviewed and approved by the Institutional Review Board of Louisiana State University Health Sciences Center New Orleans (approval #10142).
Measurements
Prostate cancer was defined according to two questions: “Have you ever been told by a doctor or other health professional that you had cancer or a malignancy of any kind?” and “What kind of cancer was it?” Subjects with prostate cancer were identified based on the primary cancer site. Additionally, subjects having more than one cancer type were excluded.
Smoking status was divided into never smoker, former smoker, and current smoker in this study, according to the detailed questions as following: “Have you smoked at least 100 cigarettes in your entire life?” and “Do you now smoke cigarettes?” Never smoker consisted of respondents who reported that they smoked fewer than 100 cigarettes in their lifetime. Former smoker consisted of respondents who reported smoking ≥100 cigarettes in their lifetime, and currently, do not smoke cigarettes. Current smoker consisted of respondents who reported smoking ≥100 cigarettes in their entire lifetime and were currently smoking every day or some days (29).
Data of the neutrophils number (1,000 cell/µL) and the lymphocyte number (1,000 cells/µL) were obtained from complete blood count measurement, which were acquired from the laboratory data in the NHANES datasets (30). NLR is divided by the neutrophils number (1,000 cell/µL) to the lymphocyte number (1,000 cells/µL). The binary NLR [low (<3) and high (≥3)] based on the NLR mean of 2.7 in this study was used for further analyses (31).
The participants’ demographic characteristics included age, race/ethnicity (non-Hispanic white, non-Hispanic black, and others), education (≤high school graduate and >high school graduate) and family poverty income ratio (PIR) (≤1, and >1). PIR≤1 indicates that the family income is below the official definition of poverty. Body mass index (BMI), number of comorbidities, and years after cancer diagnosis were also considered. BMI was calculated by body weight in kilogram and height in square meter from anthropometry measurements. BMI was classified as underweight/normal and overweight/obesity, respectively (30). The number of comorbidities, which included asthma, gout, heart failure, coronary heart disease, angina/angina pectoris, heart attack, stroke, emphysema, thyroid problem, chronic bronchitis, and any liver condition, were calculated from medical condition questionnaire. The years after cancer diagnosis was defined as the period from the age when prostate cancer was first diagnosed [“How old (were you/was SP) when prostate cancer was first diagnosed?”] to age in years at screening (“Age in years of the participant at the time of screening.”).
Statistical analysis
The prostate cancer survivors’ demographic, behavioral, and clinical characteristics were summarize using descriptive statistics by the binary NLR status. The associations of these characteristics with NLR were examined using the Rao-Scott chi-square with an adjusted F statistic for the categorical factors, and t-tests were used to examine the difference for continuous variables. An appropriate sampling weight was calculated based on the guideline (32). All descriptive analyses were performed with survey weighted commends of SAS (PROC SURVEYFREQ and PROC SURVEYMEANS) using the methods for the multistage and unequal sampling weights of this survey to display percentages (%), means, and standard errors. Sampling weighted logistic regression models (using PROC SURVEYLOGISTIC in SAS) were applied to examine the association between smoking status and NLR after adjusting potential confounders, including age, race, BMI, comorbidities, PIR, and years after cancer diagnosis. All variables had no multicollinearity in our model. The weighting analytical methods followed the instructions provided on the CDC website (33). All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC). All tests were 2-sided, and a P value <0.05 was defined as statistically significant for all tests.
Results
There were a total of 354 men adults aged ≥20 years old with prostate cancer in the NHANES 2005–2016. Table 1 provides the demographics and smoking status by the two NLR groups [high (≥3) vs. low (<3)]. For the overall study population, 33.2% (n=111) had high NLR, and 9.3% were current smokers. Prostate cancer survivors with high NLR were older (mean, 73.5 vs. 69.6 years old) and had longer years after diagnosis (mean, 8.8 vs. 6.3 years) than those who with low NLR. In addition, the rates of high NLR (≥3) were significantly higher for non-Hispanic white (38.5%), and high income (PIR >1, 34.7%) than others. The smoking status, education, BMI, and comorbidity did not have a significant impact on NLR.
Table 1
| Characteristic | Overall | NLR <3 | NLR ≥3 | P value† |
|---|---|---|---|---|
| Total, n (%) | 354 (100.0) | 243 (66.8) | 111 (33.2) | − |
| NLR, mean ± SE | 2.7±0.08 | 2.0±0.03 | 4.3±0.09 | <0.001 |
| Smoking status, n (%) | 0.927 | |||
| Never smoker | 136 (36.6) | 99 (67.1) | 37 (32.9) | |
| Former smoker | 177 (54.1) | 119 (66.1) | 58 (33.9) | |
| Current smoker | 41 (9.3) | 25 (69.9) | 16 (30.1) | |
| Age, mean ± SE (years) | 70.9±0.6 | 69.6±0.7 | 73.5±0.8 | <0.001 |
| Race/ethnicity, n (%) | <0.001 | |||
| Non-Hispanic White | 172 (71.2) | 98 (61.5) | 74 (38.5) | |
| Non-Hispanic Black | 127 (17.5) | 106 (82.9) | 21 (17.1) | |
| Others | 55 (11.3) | 39 (75.1) | 16 (24.9) | |
| Education, n (%) | 0.092 | |||
| ≤High school graduate | 165 (36.5) | 115 (67.8) | 50 (32.2) | |
| >High school graduate | 189 (63.5) | 128 (66.2) | 61 (33.8) | |
| Poverty income ratio, n (%) | 0.016 | |||
| ≤1 | 46 (8.2) | 38 (83.5) | 8 (16.5) | |
| >1 | 308 (91.8) | 205 (65.3) | 103 (34.7) | |
| BMI, n (%) | 0.328 | |||
| Underweight/normal | 89 (23.6) | 58 (61.9) | 31 (38.1) | |
| Overweight/obese | 265 (76.4) | 185 (68.3) | 80 (31.7) | |
| Comorbidity, mean ± SE | 0.8±0.1 | 0.7±0.1 | 0.8±0.1 | 0.639 |
| Year after diagnosis, mean ± SE | 7.1±0.5 | 6.3±0.4 | 8.8±0.8 | <0.001 |
†, test using Rao-Scott chi-square test or t-test. NLR, neutrophil lymphocyte ratio; BMI, body mass index; SE, standard error; NHANES, National Health and Nutrition Examination Survey.
The results of weighted logistic regression are presented in Table 2. As a crude result, older prostate cancer survivors were more likely to have high NLR [odds ratio (OR) in a 1-year increment =1.07, 95% CI: 1.03–1.10]. Prostate cancer survivors who are non-Hispanic blacks, compared with non-Hispanic whites, were significantly associated with low NLR (OR=0.33, 95% CI: 0.19–0.57). Prostate cancer survivors with PIR>1 were more likely to have a high NLR than those with PIR≤1 (OR=2.70, 95% CI: 1.16–6.28). Longer years after diagnosis was associated with high NLR (OR in a 1-year increment =1.05, 95% CI: 1.01–1.10). For evaluating smoking status associated with NLR adjusting for the selected factors, the multivariable weighted logistic regression models were conducted. After controlling for the selected factors, older prostate cancer survivors were more likely to have high NLR (adjusted OR in a 1-year increment =1.05, 95% CI: 1.01–1.09). Race/ethnicity was significantly associated with NLR level. Non-Hispanic blacks (vs. non-Hispanic whites) were less likely to have high NLR (adjusted OR=0.42, 95% CI: 0.23–0.75). Although the main effect of cigarette smoking was not statistically significant associated with NLR, the interaction effect of smoking status and race/ethnicity associated with the NLR level was significant (P=0.040).
Table 2
| Characteristics | Unadjusted OR | 95% CI | Adjusted OR | 95% CI |
|---|---|---|---|---|
| Smoking status | ||||
| Never smoker (Ref) | 1.00 | 1.00 | ||
| Former smoker | 1.05 | 0.57–1.92 | 0.97 | 0.52–1.82 |
| Current smoker | 0.88 | 0.41–1.87 | 1.38 | 0.50–3.84 |
| Age (years) | 1.07*** | 1.03–1.10 | 1.05** | 1.01–1.09 |
| Race/ethnicity | ||||
| Non-Hispanic white (Ref) | 1.00 | 1.00 | ||
| Non-Hispanic black | 0.33* | 0.19–0.57 | 0.42* | 0.23–0.75 |
| Others | 0.53 | 0.26–1.06 | 0.64 | 0.31–1.31 |
| Education | ||||
| ≤High school graduate (Ref) | 1.00 | 1.00 | ||
| >High school graduate | 1.07 | 0.58–1.99 | 1.19 | 0.61–2.32 |
| Poverty income ratio | ||||
| ≤1 (ref) | 1.00 | 1.00 | ||
| >1 | 2.70* | 1.16–6.28 | 2.23 | 0.91–5.47 |
| BMI | ||||
| Underweight/normal (Ref) | 1.00 | 1.00 | ||
| Overweight/obese | 0.76 | 0.43–1.33 | 1.02 | 0.56–1.85 |
| Comorbidity (numbers) | 1.02 | 0.82–1.26 | 0.99 | 0.80–1.24 |
| Year after diagnosis (years) | 1.05* | 1.01–1.10 | 1.03 | 0.99–1.07 |
All variables had no multicollinearity in the adjust model. Interaction term (smoking status and race/ethnicity) had a statistical significance (P=0.040). *P<0.05; **P<0.01; ***P<0.001. NLR, neutrophil lymphocyte ratio; BMI, body mass index; OR, odds ratio; CI, confidence interval.
Figure 1 provides the effect of smoking status on NLR levels in prostate cancer survival patients stratified by race/ethnicity. In non-Hispanic black prostate cancer survivors, current smokers were found significantly increased NLR levels than never smokers (OR =3.69, 95% CI: 1.36–9.99). However, similar results (current smoker vs. never smoker) were not observed in non-Hispanic whites (OR =0.99, 95% CI: 0.26–3.79) and other races (OR =0.51, 95% CI: 0.09–2.87). The details of the relationships between smoking status, race/ethnicity, and high NLR level were shown in Table 3. Among non-Hispanic black prostate cancer survivors, current smokers tended to have a high NLR compared with the never smokers (36.9% vs. 13.7%). However, similar rates of a high NLR were observed among the three various smoking groups among the non-Hispanic white prostate cancer survivors (33.6–39.0%).
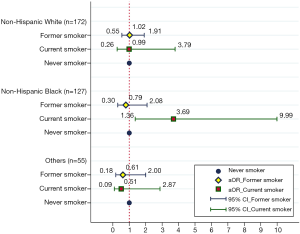
Table 3
| Smoking status | High NLR (%) | ||
|---|---|---|---|
| Non-Hispanic white (n=172) | Non-Hispanic black† (n=127) | Other races (n=55) | |
| Never smoker | 38.4 | 13.7 | 32.5 |
| Former smoker | 39.0 | 11.4 | 21.8 |
| Current smoker | 33.6 | 36.9 | 15.1 |
†, test using Rao-Scott chi-square test, P<0.01. NLR, neutrophil lymphocyte ratio.
Discussion
This study used the NHANES data to examine the association between smoking status and NLR levels among prostate cancer survivors in the U.S. The findings of this study revealed that cigarette smoking was significantly associated with an elevated NLR among non-Hispanic black prostate cancer survivors. However, the significant association was not observed in non-Hispanic whites and other races.
In this study, the average age of prostate cancer survivors was 70.9 years old, and increased age was observed to be associated with high NLR (OR =1.05). Previous studies have illustrated that aging is related to increased levels in inflammatory markers such as NLR (34). Inflammation causes morbidity and early death in the elderly. Therefore, older prostate cancer survivors should avoid hazardous behaviors such as smoking to reduce the risk of elevated NLR. This study’s findings also demonstrated that non-Hispanic black prostate cancer survivors were less likely to have high NLR than non-Hispanic whites (17.1% vs. 38.5%, P<0.001). This trend of NLR is supported by a previous study that utilized the 2007–2010 NHANES database to examine the average values of NLR of the general population and the racial differences. Non-Hispanic black had significantly lower mean NLR values (NLR=1.76) as compared to non-Hispanic whites (NLR=2.2) (11).
Our study further evaluated the interaction effect of smoking status and race/ethnicity on NLR level (P=0.040). After stratifying by race/ethnicity, 36.9% non-Hispanic black prostate cancer survivors who are current smokers with a high NLR level were identified in this study (11.4% in former smoker and 13.7% in never smoker, respectively). Higher smoking rate in non-Hispanic black prostate cancer survivors was supported by prior research, which showed African Americans who are a prostate cancer survivor were more likely to be current smokers (17%) (35). Additionally, cigarette smoking’s role in elevated NLR has been demonstrated in previous studies. One study reported higher levels of NLR in smokers as compared to nonsmokers among healthy participants (9). A cross-sectional study also found that cigarette smokers’ NLR levels were superior as compared to the non-smoking group among healthy adults, aged between 18 and 40 years (25). Fest and colleagues reported that smokers presented higher NLR than never smokers, which also increased the risk of mortality for smokers among the aging population (26). Moreover, NLR levels have been proven to be associated with cancer progression among cancer survivors. Shafique et al. reported higher NLR was associated with the worse 5-year relative survival among prostate cancer patients (14). Langsenlehner et al. demonstrated that elevated basal NLR as an independent prognostic marker, which is associated with decreased prostate cancer survival (15). Meta-analysis studies have also confirmed the negative association between NLR and prostate cancer prognosis, recurrence, and overall survival (16-18). Therefore, non-Hispanic black prostate cancer survivors who are current smokers should be encouraged to quit smoking. Smoking cessation might result in reduced levels of NLR, thereby improving their survival rate. Additionally, future studies are encouraged to investigate the association among other types of cancer survivors.
In terms of cutoff points for NLR, there are different definite NLR cutoff points being applied. Previous studies investigating NLR have determined different cutoff values for elevation, ranging from 2 to 5 (17,31,36). In the present study, a cutoff point of 3 was used to define high and low NLR levels because of data distribution and previous literature (31). We also conducted analyses using each group’s average of NLR (2.9 for non-Hispanic whites, 2.1 for non-Hispanic blacks, and 2.5 for others) and a cutoff point of 5, which were observed the similar trend with a cutoff point of 3. However, to our knowledge, there is no consist conclusion to point out the optimal cutoff point for NLR. Future studies could conduct more investigations in the cutoff point for NLR.
There are limitations to this study. Because self-reported cancer status was used in this study, bias might be present. Next, we combined six consecutive 2-year survey cycle datasets to improve the statistical reliability of the estimate; however, the combination might include rare overlapped participants (30). Additionally, the NLR measurements among prostate cancer survivors may not be generalizable to the entire prostate cancer population because hospitalized prostate cancer patients were not considered. Moreover. this study excluded cancer survivors with multiple cancer types, which may also limit the generalizability of our study findings. Finally, this study cannot identify or establish a causal relationship between smoking status and NLR due to the cross-sectional nature of the survey data.
Although limitations exist, this study has several strengths. The NHANES data is highly representative of the entire U.S. population. Further, the present study’s findings supported the association between cigarette smoking and NLR level among non-Hispanic black prostate cancer survivors, even when controlling for demographic characteristics, and medical conditions. The findings provide more information than previous studies examining only among non-Hispanic white or Caucasian populations (17).
Conclusions
This study indicated non-Hispanic black prostate cancer survivors who currently smoked were more likely to have high NLR levels than never smokers. We recommend that intervention programs that aim to provoke smoking cessation among prostate cancer survivors should primarily focus on non-Hispanic black population. In addition, non-Hispanic black prostate cancer survivors who smoke should be encouraged to stop smoking, as this might benefit prostate cancer management and reduce the risk of cancer progression.
Acknowledgments
Funding: This research was supported by
Footnote
Provenance and Peer Review: The article was commissioned by the editorial office, Translational Cancer Research for the series “Population Science in Cancer”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.05.23). The series “Population Science in Cancer” was commissioned by the editorial office without any funding or sponsorship. HYL served as the unpaid Guest Editor of the series and serves as the unpaid editorial board members of Translational Cancer Research from Jul 2018 to Jun 2020. TST served as the unpaid Guest Editor of the series and serves as the unpaid editorial board members of Translational Cancer Research from Jan 2019 to Dec 2020. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent was signed by each participant before conducting the data collection. The present study was reviewed and approved by the Institutional Review Board of Louisiana State University Health Sciences Center New Orleans (approval #10142).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- National Cancer Institute Surveillance, Epidemiology, and End Results Program (US). Cancer state facts: Prostate cancer. 2019 Jan. Available online: https://seer.cancer.gov/statfacts/
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Gong Z, Agalliu I, Lin DW, et al. Cigarette smoking and prostate cancer-specific mortality following diagnosis in middle-aged men. Cancer Causes Control 2008;19:25-31. [Crossref] [PubMed]
- Murta-Nascimento C, Romero AI, Sala M, et al. The effect of smoking on prostate cancer survival: a cohort analysis in Barcelona. Eur J Cancer Prev 2015;24:335-9. [Crossref] [PubMed]
- Polesel J, Gini A, Dal Maso L, et al. The negative impact of tobacco smoking on survival after prostate cancer diagnosis. Cancer Causes Control 2015;26:1299-305. [Crossref] [PubMed]
- Darcey E, Boyle T. Tobacco smoking and survival after a prostate cancer diagnosis: A systematic review and meta-analysis. Cancer Treat Rev 2018;70:30-40. [Crossref] [PubMed]
- De Nunzio C, Andriole GL, Thompson IM Jr, et al. Smoking and prostate cancer: a systematic review. Eur Urol Focus 2015;1:28-38. [Crossref] [PubMed]
- Islami F, Moreira DM, Boffetta P, et al. A Systematic Review and Meta-analysis of Tobacco Use and Prostate Cancer Mortality and Incidence in Prospective Cohort Studies. Eur Urol 2014;66:1054-64. [Crossref] [PubMed]
- Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860. [Crossref] [PubMed]
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001;357:539-45. [Crossref] [PubMed]
- Azab B, Camacho-Rivera M, Taioli E. Average Values and Racial Differences of Neutrophil Lymphocyte Ratio among a Nationally Representative Sample of United States Subjects. PLoS One 2014;9:e112361. [Crossref] [PubMed]
- Guthrie GJK, Charles KA, Roxburgh CSD, et al. The systemic inflammation-based neutrophil–lymphocyte ratio: Experience in patients with cancer. Crit Rev Oncol Hematol 2013;88:218-30. [Crossref] [PubMed]
- Templeton AJ, McNamara MG, Šeruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 2014;106:dju124. [Crossref] [PubMed]
- Shafique K, Proctor MJ, McMillan DC, et al. Systemic inflammation and survival of patients with prostate cancer: evidence from the Glasgow Inflammation Outcome Study. Prostate Cancer Prostatic Dis 2012;15:195-201. [Crossref] [PubMed]
- Langsenlehner T, Thurner EM, Krenn-Pilko S, et al. Validation of the neutrophil-to-lymphocyte ratio as a prognostic factor in a cohort of European prostate cancer patients. World J Urol 2015;33:1661-7. [Crossref] [PubMed]
- Yin X, Xiao Y, Li F, et al. Prognostic role of neutrophil-to-lymphocyte ratio in prostate cancer: a systematic review and meta-analysis. Medicine 2016;95:e2544. [Crossref] [PubMed]
- Gu X, Gao X, Li X, et al. Prognostic significance of neutrophil-to-lymphocyte ratio in prostate cancer: evidence from 16,266 patients. Sci Rep 2016;6:22089. [Crossref] [PubMed]
- Wang Z, Peng S, Xie H, et al. Neutrophil–lymphocyte ratio is a predictor of prognosis in patients with castration-resistant prostate cancer: a meta-analysis. Cancer Manag Res 2018;10:3599. [Crossref] [PubMed]
- Williams KA, Labidi-Galy SI, Terry KL, et al. Prognostic significance and predictors of the neutrophil-to-lymphocyte ratio in ovarian cancer. Gynecol Oncol 2014;132:542-50. [Crossref] [PubMed]
- Sarraf KM, Belcher E, Raevsky E, et al. Neutrophil/lymphocyte ratio and its association with survival after complete resection in non–small cell lung cancer. J Thorac Cardiovasc Surg 2009;137:425-8. [Crossref] [PubMed]
- Azab B, Bhatt VR, Phookan J, et al. Usefulness of the neutrophil-to-lymphocyte ratio in predicting short-and long-term mortality in breast cancer patients. Ann Surg Oncol 2012;19:217-24. [Crossref] [PubMed]
- Sharaiha RZ, Halazun KJ, Mirza F, et al. Elevated preoperative neutrophil: lymphocyte ratio as a predictor of postoperative disease recurrence in esophageal cancer. Ann Surg Oncol 2011;18:3362-9. [Crossref] [PubMed]
- Gomez D, Farid S, Malik H, et al. Preoperative neutrophil-to-lymphocyte ratio as a prognostic predictor after curative resection for hepatocellular carcinoma. World J Surg 2008;32:1757-62. [Crossref] [PubMed]
- Gumus F, Solak I, Eryilmaz M. The effects of smoking on neutrophil/lymphocyte, platelet//lymphocyte ratios. Bratisl Lek Listy 2018;119:116-9. [PubMed]
- Kumari B, Aslam SK, Zaheer S, et al. Systemic inflammatory markers among waterpipe smokers, cigarette smokers, and nonsmokers. J Addict Med 2019;13:55-60. [Crossref] [PubMed]
- Fest J, Ruiter TR, Koerkamp BG, et al. The neutrophil-to-lymphocyte ratio is associated with mortality in the general population: The Rotterdam Study. Eur J Epidemiol 2019;34:463-70. [Crossref] [PubMed]
- Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey. Available online: http://www.cdc.gov/nchs/nhanes.htm
- Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey. NCHS Research Ethics Review Board (ERB) Approval. Available online: https://www.cdc.gov/nchs/nhanes/irba98.htm
- McClave AK, Dube SR, Strine TW, et al. Associations between health-related quality of life and smoking status among a large sample of US adults. Prev Med 2009;48:173-9. [Crossref] [PubMed]
- Centers for Disease Control and Prevention. Continuous NHANES. Available online: https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx
- Lorente D, Mateo J, Templeton AJ, et al. Baseline neutrophil-lymphocyte ratio (NLR) is associated with survival and response to treatment with second-line chemotherapy for advanced prostate cancer independent of baseline steroid use. Ann Oncol 2015;26:750-5. [Crossref] [PubMed]
- Centers for Disease Control and Prevention. NHANES survey methods and analytic guidelines. Available online: https://wwwn.cdc.gov/nchs/nhanes/analyticguidelines.aspx
- Centers for Disease Control and Prevention. Specifying weighting parameters. Available online: https://www.cdc.gov/nchs/tutorials/nhanes/surveydesign/weighting/intro.htm
- Fest J, Ruiter R, Ikram MA, et al. Reference values for white blood-cell-based inflammatory markers in the Rotterdam Study: a population-based prospective cohort study. Sci Rep 2018;8:10566. [Crossref] [PubMed]
- Atere-Roberts J, Hall IJ, Smith JL. Racial and ethnic disparities in comorbidities and behavioral risk factors among prostate cancer survivors, NHIS 2015. Cancer Epidemiology Biomarkers & Prevention 2018;27:C37.
- Wei Y, Jiang YZ, Qian WH. Prognostic role of NLR in urinary cancers: a meta-analysis. PLoS One 2014;9:e92079. [Crossref] [PubMed]


