
The correlation of ESCO1 expression with a prognosis of prostate cancer and anti-tumor effect of ESCO1 silencing
Introduction
Prostate cancer is one of the most common types of malignant tumor among adult males in Western societies (1). In China, although the incidence of prostate cancer is lower compared with that in Western countries, it has increased in recent years, particularly in metropolitan areas (2). With the aging of the Chinese population, prostate cancer is becoming a serious public health issue in China. Current treatments for prostate cancer usually exhibit variable efficiencies and eventually lead to drug-resistance and metastasis. Thus, further studies investigating the molecular mechanism underlying prostate cancer progression through the identification of essential molecules involved in this process are warranted. This will facilitate the development of more effective strategies to treat patients with prostate cancer.
Establishment of sister chromatid cohesion N-acetyltransferase 1 (ESCO1) is an acetyltransferase essential for the establishment of sister chromatid cohesion (3-6). Sister chromatid cohesion serves a vital role in mitosis and is essential for the maintenance of genome integrity by regulating correct chromosome segregation (7). Defects in this process may lead to tumorigenesis or aneuploidy (8). As cohesion is also essential for recombinational repair, the dysfunction of ESCO1 may result in a high proportion of improperly repaired chromosomes (9). These phenomena are all associated with tumorigenesis. Therefore, ESCO1 may be of importance in the development and progression of cancer.
Certain major endometrial cancer histotypes are characterized by aneuploidy and chromosome instability (10,11). Price et al. (10) sequenced 21 candidate chromosome instability genes to search for nucleotide variants and identify non-synonymous somatic mutations in ESCO1. This suggests that ESCO1 is essential for endometrial cancer development. ESCO1, and another DNA repair gene, DNA polymerase iota, have been proposed as susceptibility genes for transmembrane protease serine 2 (TMPRSS2)-ETS transcription factor (ERG) fusion-positive prostate cancer (11). These studies suggest the critical role ESCO1 may have in tumor development and progression. However, the function of ESCO1 in prostate cancer remains unclear.
To investigate the role of ESCO1 in prostate cancer progression, its expression was analyzed in benign prostatic hyperplasia (BPH) and prostate cancer tissue samples, and its association with the pathological behavior of prostate cancer cells in vitro and in vivo was examined. To the best of our knowledge, the present study is the first to investigate the role of ESCO1 in prostate cancer progression and the possible underlying molecular mechanisms of its action.
Methods
Patients and samples
The present study was performed in accordance with a protocol approved by the Ethics Committee of the Air Force General Hospital (Beijing, China). Written informed consent was received from all patients. Prostate cancer tissue samples from 114 patients (mean age, 64 years; range, 43–85 years) who were hospitalized between January 2002 and January 2012 were collected from the Air Force General Hospital. None of these patients had received any preoperative therapy. A total of 8 metastatic cancer and 6 BPH tissue samples, serving as controls, were also obtained. Patient clinicopathological data are presented in Table 1. Clinical follow-up data were last updated in 2012. The median follow-up time was 68.9 months (range, 1–120 months). The 114 samples were all obtained at the time of radical prostatectomy from patients with clinically localized prostate cancer. The 8 metastatic samples were obtained from distinct tissues, including from the lymph nodes, bones, and lungs. None of these patients had previously undergone surgery, anti-androgenic therapy, or other treatments. Tumors were graded using the Gleason scoring system (12) and the tumor-node-metastasis (TNM) grading system (13). The pathological diagnoses were determined by two experienced urological pathologists who were blinded to the clinical information of patients.
Table 1
| Parameter | ESCO1 expression | P value | |
|---|---|---|---|
| High | Low | ||
| Age, years | 0.311 | ||
| <60 | 9 | 11 | |
| ≥60 | 54 | 40 | |
| Lymph node metastasis | 0.012* | ||
| Absence | 44 | 46 | |
| Presence | 19 | 5 | |
| Surgical margin status | 0.872 | ||
| Absence | 55 | 44 | |
| Presence | 8 | 7 | |
| Seminal vesicle invasion | 0.822 | ||
| Absence | 56 | 46 | |
| Presence | 7 | 5 | |
| PSA, ng/mL | 0.001* | ||
| ≤10 | 4 | 5 | |
| 10-20 | 35 | 11 | |
| >20 | 24 | 35 | |
| TNM stage | 0.026* | ||
| I | 28 | 33 | |
| II | 23 | 16 | |
| III | 5 | 1 | |
| IV | 7 | 1 | |
| Gleason score | <0.001* | ||
| <7 | 1 | 10 | |
| 7 (3+4) | 1 | 19 | |
| 7 (4+3) | 2 | 12 | |
| >7 | 59 | 10 | |
| Biochemical recurrence | <0.001* | ||
| Absence | 15 | 8 | |
| Presence | 48 | 43 | |
*, indicates a statistically significant result. ESCO1, establishment of sister chromatid cohesion N-acetyltransferase 1; PSA, prostate-specific antigen.
Immunohistochemical analysis
Immunohistochemical analysis was performed to detect the expression of ESCO1 in BPH, human prostate cancer, and metastasis tissue samples. Formalin-fixed paraffin-embedded tissue was cut into 4-µm-thick sections. Following deparaffinization, citrate buffer was applied for antigen retrieval (20 min, 98 °C), and 3% H2O2 was used to quench endogenous peroxidase activity at room temperature for 5 min. The sections were rinsed with PBS and incubated with 10% normal goat serum (Beijing Zhongshan Jinqiao Biotechnology Co., Ltd., Beijing, China) at room temperature for 10 min. Subsequently, sections were washed with PBS and incubated overnight at 4 °C with anti-ESCO1 antibody (catalog no., H00114799-B01P, MaxPab, USA; dilution, 1:200). Following incubation with a horseradish peroxidase (HRP)-conjugated rabbit anti-mouse IgG secondary antibody at room temperature (Beijing Zhongshan Jinqiao Biotechnology Co., Ltd.; catalog no. ZM-0468; dilution, 1:50) for 1 h, 3,3’-diaminobenzidine (Beijing Zhongshan Jinqiao Biotechnology Co., Ltd.) was used for signal visualization. For expression intensity analysis, staining was scored as follows: 0, no staining; 1, weak; 2, medium; and 3, strong. Limited by the number of negative patients (only 7), samples scored of 0 or 1 were considered the low expression, while scored of 2 or 3 were considered as high expression. A microscope (Leica DM4000B/M; Leica Microsystems, Inc., Buffalo Grove, IL, USA; magnification, ×200) was used to record the results.
Cells and transfection
Human prostate cancer cell lines PC3 and Du145 (American Type Culture Collection, Manassas, VA, USA) were cultured in RPMI-1640 medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS; Hangzhou Sijiqing Biological Engineering Materials Co., Ltd., Hangzhou, China), and incubated at 37 °C with 5% CO2. Short hairpin (sh)RNA-targeting ESCO1 (5'-CGATAAGAATTCAGAAACAGA-3') was inserted into a pUCTP vector (3D Medicines Co., Ltd., Shanghai, China; http://www.3dmedcare.com/) and packaged into lentiviruses. PC3 and DU145 cells were transfected with 8 µg/mL Polybrene (Rongbai, Shanghai, China). All procedures were performed according to the manufacturer’s protocol (3D Medicines Co., Ltd.).
Cell viability assay
Following culture for 24 h in 100 µL complete medium (RPMI-1640 with 10% FBS) in a 96-well plate, PC3 and Du145 cells (4×103) were transfected with shRNA lentivirus for 72 h at 37 °C. Subsequently, 20 µL MTS (Cell Titer 96® Aqueous One Solution Cell Proliferation Assay; Promega Corporation, Madison, WI, USA) was added to each well, and the cells were incubated at 37 °C for 4 h. The absorbance was examined using an ELISA reader at a wavelength of 490 nm according to the protocol of the MTS manufacturer. The experiments were performed at least twice in triplicate.
Cell migration and invasion assays
Cell migratory and invasive abilities were determined using a Transwell assay (Corning Incorporated, Corning, NY, USA) with a pore size of 8 µm 72 h following infection. For the cell migration assay, 1×105 cells in 100 µL medium were seeded into the upper chamber. After 24 h of incubation at 37 °C, cells in the upper chamber were removed. The cells that traversed the membrane were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet. For the cell invasion assay, 1×105 cells were seeded into the upper compartment of the Transwell inserts in serum-free RPMI-1640 medium. After 24 h of incubation at 37 °C, the non-invading cells were removed from the top of the Matrigel. Invasive cells at the bottom were fixed in 4% paraformaldehyde and stained with 0.1% crystal violet. For quantification, cells in six random fields were counted under a light microscope. Each experiment was repeated in triplicate.
Cell cycle analysis
Control and lentiviral shRNA-infected cells were harvested with trypsin-EDTA (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) 72 h after infection (4 °C, 12,000× g, 5 min), washed with PBS, and then fixed with 70% ethanol. Centrifuged pellets were resuspended in 100 µL hypotonic citric buffer (192 mmol/L Na2HPO4 and 4 mmol/L citric acid), incubated for 30 min at room temperature, centrifuged again (4 °C, 12,000× g, 5 min), and suspended in propidium iodide (PI)/RNase/PBS (100 µg/mL PI and 10 µg/mL RNase A) overnight at 4 °C. Cell cycle analysis was performed using the FACSCalibur™ system (BD Biosciences, San Jose, CA, USA) and BD FACStation software© 2007 (BD Biosciences), according to the manufacturer’s protocol.
Western blot analysis
After 74 h of infection, cells from the control and lentiviral shRNA infection groups were resuspended in 1X SDS loading buffer with protease inhibitors (Guduobio, Shanghai, China) and lysed. The bicinchoninic acid method was used for protein determination. PMSF was used for protein extraction. The lysates (20 µg/lane) were analyzed using 12% SDS-PAGE and transferred to nitrocellulose membranes. Non-fat milk (5%) was used for blocking for 10 min at room temperature. Antibodies directed against GAPDH (cat. no., M20028; Abmart, Shanghai, China; dilution, 1:200), protein kinase B (Akt), and phosphorylated Akt (cat. nos. sc-24500 and sc 7985-R, respectively; Santa Cruz Biotechnology, Inc., Dallas, TX, USA; dilution, 1:1,000) were used and incubated overnight at 4 °C for immunoblotting, followed by HRP-conjugated secondary anti-mouse (catalog no. 170-6516; dilution, 1:100; Bio-Rad Laboratories, Inc., Hercules, CA, USA) or anti-rabbit antibody (catalog no. SSA018; dilution, 1:150; Sino Biological, Inc., Beijing, China) which were incubated at room temperature for 1 h. Visualization and analysis were performed by using a chemiluminescence detection system (Pierce; Thermo Fisher Scientific, Inc.) and quantified relative to GAPDH in ImageJ (v2.1.4.7, National Institutes of Health, Bethesda, MD, USA).
Subcutaneous in vivo experiments
PC3 cells and lentiviral shRNA-infected cells (1×106 cells/flank) in 100 µL PBS/Matrigel (50:50) were subcutaneously injected into 5 male nude mice (8 weeks old, 18–24 g), respectively. The mice were housed in a temperature-controlled room at 25 °C with a relative humidity of 50%±10%, which was artificially kept on a 12-h light/12-h dark schedule. Sawdust was used as bedding and changed once every 2 days. Tap-water and pelleted rat feed were available ad libitum. The mice were monitored daily. The body weight and tumor diameters of each mouse were determined weekly. Mice were sacrificed at 4 weeks post-implantation, and tumors were excised and weighed. All procedures were performed in accordance with the institutional ethical requirements of the Air Force General Hospital and approved by the Ethics Committee of Air Force General Hospital.
Statistical analysis
The association between ESCO1 expression and prostate-specific antigen (PSA) level, TNM stage, Gleason score, and the nature of prostate tissue samples (benign, malignant, or metastatic) was evaluated using the Pearson’s χ2 or two-sided Fisher’s exact test. Kaplan-Meier estimator curves were used to analyze survival across categories of ESCO1 expression levels. The differences between the patient groups were assessed using the log-rank test. Cox’s proportional hazards regression analysis was performed to test the statistical independence of ESCO1 deletions. Data are presented as the mean ± standard deviation. P<0.05 was considered to indicate a statistically significant difference. All statistical analyses were performed using SPSS software (version 18.0; SPSS, Inc., Chicago, IL, USA).
Results
ESCO1 expression is upregulated in human prostate cancer and metastasis tissue
A total of 114 archived prostate cancer, 8 metastatic tumor, and 6 BPH samples were analyzed for ESCO1 expression using immunohistochemical analysis. Among the 114 prostate cancer samples, 7 (6.1%) were negative for ESCO1, and 43 (37.7%) were weakly, 33 (28.9%) were moderately, and 31 (27.2%) were strongly stained. Patients were stratified by ESCO1 expression. According the expression level of ESCO1, cases were divided into a low-ESCO1 group (negative/weak) and a high-ESCO1 group (moderate/strong). Thus, among the 114 prostate cancer samples, 51 tumors (44.7%) were classified as low-ESCO1 group, and 63 tumors (55.3%) were high-ESCO1 group. The association between ESCO1 expression and clinicopathological characteristics are presented in Table 1. ESCO1 was expressed at higher levels in metastatic prostate cancer (all 8 samples exhibited marked ESCO1 expression) compared with in localized prostate tumor and in BPH tissue (all 6 BPH samples were negative) (Figure 1A,B,C,D). The results also demonstrated that cancer tissue samples with a high Gleason score exhibited increased ESCO1 expression compared with samples with a low Gleason score (Figure 1B,C). Furthermore, there was an increased level of ESCO1 expression in the tumor compared within the BPH tissue. These results provide the first evidence of an association between ESCO1 expression and malignancy and metastasis of prostate cancer.
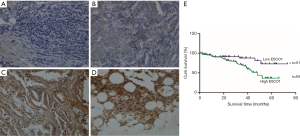
Increased ESCO1 expression is associated with poor patient survival
Since the TNM stage, PSA level, and other clinical parameters are important prognostic indicators for patients with prostate cancer, the association between ESCO1 expression and these clinical parameters of prostate cancer was investigated. ESCO1 staining was found to be significantly associated with lymph node metastasis, PSA level, TNM stage, biochemical recurrence, and Gleason score (Table 1). The high ESCO1 expression group exhibited significantly decreased overall survival times compared with the low ESCO1 expression group (log-rank test, P=0.009; Figure 1E). The median survival time was 29 months for patients with high ESCO1 expression and 41 months for patients with low ESCO1 expression. Using Cox’s proportional hazards model, the prognostic value of the following factors was analyzed: patient age, PSA level, lymph node metastasis, surgical margin status, seminal vesicle invasion, Gleason score, TNM stage, biochemical recurrence, and ESCO1 expression (Table 2). ESCO1 was identified to be a strong independent predictor for patient overall survival [hazard ratio (HR), 2.948; 95% confidence interval (CI), 1.287–6.755; P=0.011] (Table 2). Taken together, these results suggest that ESCO1 may be a useful prognostic marker for patients with prostate cancer.
Table 2
| Prognosis factors | HR | 95% CI | P value |
|---|---|---|---|
| Age, years | |||
| <60 | 1 | ||
| ≥60 | 1.259 | 0.703–2.255 | 0.439 |
| Lymph node metastasis | |||
| Absence | 1 | ||
| Presence | 1.962 | 0.781–4.935 | 0.151 |
| Surgical margin status | |||
| Absence | 1 | ||
| Presence | 1.186 | 0.497–2.831 | 0.700 |
| Seminal vesicle invasion | |||
| Absence | 1 | ||
| Presence | 1.059 | 0.518–2.164 | 0.876 |
| PSA, ng/mL | |||
| ≤10 | 1 | ||
| 10–20 | 0.514 | 0.195–1.354 | 0.178 |
| >20 | 0.924 | 0.541–1.579 | 0.773 |
| TNM stage | |||
| I | 1 | ||
| II | 1.319 | 0.392–4.439 | 0.655 |
| III | 1.283 | 0.342–4.815 | 0.712 |
| IV | 0.647 | 0.125–3.362 | 0.605 |
| Gleason score | |||
| <7 | 1 | ||
| 7 (3+4) | 1.362 | 0.311–5.513 | 0.158 |
| 7 (4+3) | 2.287 | 0.702–5.924 | 0.081 |
| >7 | 2.581 | 0.962–6.115 | 0.052 |
| Biochemical recurrence | |||
| Absence | 1 | ||
| Presence | 1.035 | 0.562–1.905 | 0.912 |
| ESCO1 expression | |||
| Low | 1 | ||
| High | 2.948 | 1.287–6.755 | 0.011* |
*, indicates a statistically significant result. HR, hazard ratio; CI, confidence interval; ESCO1, establishment of sister chromatid cohesion N-acetyltransferase 1; PSA, prostate-specific antigen.
ESCO1 knockdown inhibits prostate cancer cell viability and induces apoptosis of PC3 and DU145 cells
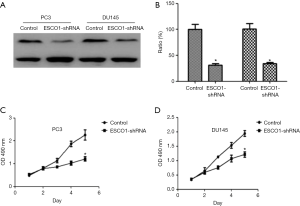
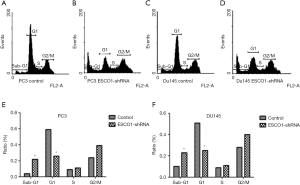
The effect of ESCO1 silencing on prostate cancer cell viability was investigated in PC3 and DU145 cell lines using an MTS assay. The downregulation of ESCO1 was confirmed through western blotting, which revealed that ESCO1-targeting shRNA decreased ESCO1 expression by 65.8% and 54.3% in PC3 and DU145, respectively (Figure 2A,B). ESCO1 knockdown demonstrated a significant inhibitory effect on the viability of prostate cancer PC3 and DU145 cells (Figure 2C,D). To address the molecular mechanism underlying this effect, the effect of ESCO1 knockdown was further investigated on cell cycle progression and apoptosis of prostate cancer cells using PI staining and flow cytometry. As presented in Figure 3, the knockdown of ESCO1 decreased the proportion of cells in G1 phase, while increasing the proportion of sub-G1 apoptotic cells. The ESCO1 silencing in PC3 and DU145 cells resulted in a 23.19% and 21.23% increase of apoptotic cells, respectively, which is a significant increase compared with the 4.66% and 5.02% increase in the control PC3 and DU145 cells, respectively (P<0.05).
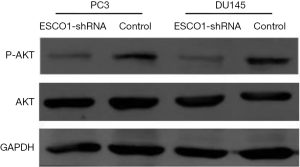
The phosphoinositide 3-kinase-Akt signaling pathway is essential for the regulation of various biological processes, including cell proliferation, cell cycle progression, apoptosis, and metastasis. The results of the western blot analysis indicated a marked decrease in Akt phosphorylation following ESCO1 silencing (Figure 4). These results suggest that ESCO1 knockdown may induce apoptosis in human prostate cancer cells.
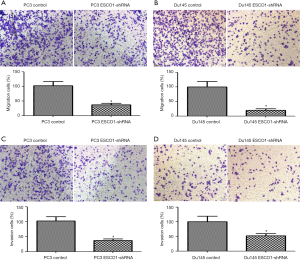
ESCO1 knockdown inhibits the migration and invasion of prostate cancer cells in vitro
Owing to the increased ESCO1 expression observed in metastasis tissue samples, it was hypothesized that ESCO1 might play an essential role in prostate cancer metastasis. To evaluate the effects of ESCO1 on cell migration, Transwell migration, and invasion assays were performed. It was revealed that ESCO1 silencing significantly inhibited the migratory ability of PC3 and DU145 cells (Figure 5A,B). In addition, cells with knocked down ESCO1 exhibited significantly lower invasive potency compared with the control cells (Figure 5C,D). These results suggest that ESCO1 serves an essential role in promoting the migratory and invasive ability of prostate cancer cells.
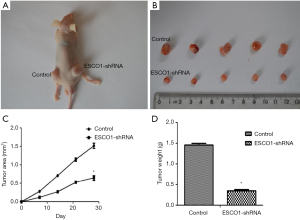
ESCO1 silencing suppresses tumor growth in vivo
The aforementioned in vitro data suggest that decreased ESCO1 expression contributes to the suppression of prostate cancer cell viability and metastasis. To investigate the in vivo effect of ESCO1 silencing on tumor growth, a subcutaneous tumor model was established in nude mice. Tumors with downregulated ESCO1 expression sustained a significant tumor growth arrest compared with that of the control group (Figure 6). Figure 6A is a representative image demonstrating tumor growth in the control and ESCO1 knockdown sides of a mouse. These results further support the significant effect of ESCO1 silencing on prostate cancer growth.
Discussion
Sister chromatid cohesion serves essential roles in chromosome segregation, genome stability maintenance, and homologous recombination repair of DNA double-strand breaks (DSBs) (7-9). Growing evidence indicates that mutational disruption of sister chromatid cohesion genes is associated with human tumor development (14-18). In glioblastoma, colorectal cancer, Ewing’s sarcoma, myeloid diseases, acute myeloid leukemia, and melanoma, a number of genes that regulate sister chromatid cohesion have been identified undergoing somatic deletions and mutations (14-16).
ESCO1 is essential to the establishment of sister chromatid cohesion. The acetyltransferase and cohesive activity of ESCO1 are essential for the response to DNA damage checkpoint and DSBs (3). ESCO1 was reported to be somatically mutated in endometrial cancer (10) and was also proposed as a susceptibility gene for TMPRSS2-ERG fusion-positive prostate cancer, therefore reinforcing the importance of ESCO1 in human malignancy (11). However, the effect of ESCO1 mutation on the development of prostate cancer and other malignancies remains unclear.
In the present study, it was demonstrated that ESCO1 expression is upregulated in prostate tumors. Its expression was identified in all prostate cancer samples, and particularly in metastatic prostate cancer. However, there was no detectable ESCO1 expression in BPH prostate tissue samples. Thus, these results suggest that ESCO1 serves a vital role in the progression and metastasis of prostate cancer.
Currently, clinicopathological factors, including Gleason scores, TNM stage, PSA level, and lymph node metastasis, are used to guide clinical treatment (19-21). In the present study, it was revealed that ESCO1 expression was significantly associated with Gleason scores, TNM stage, PSA level, lymph node metastasis, and biochemical recurrence (Table 1). To determine whether ESCO1 has any prognostic significance, Kaplan-Meier estimator survival analysis and Cox’s regression analysis were performed. Kaplan-Meier estimator survival analysis indicated that patients with increased ESCO1 expression exhibited significantly decreased overall survival times compared with the lower ESCO1 expression group (P=0.009). In the Cox’s proportional hazards model, ESCO1 was proven to be a strong independent predictor of patient overall survival (HR, 2.948; 95% CI, 1.287–6.755; P=0.011). Therefore, ESCO1 may serve as a prognostic biomarker for prostate cancer.
Furthermore, the anti-tumor effect of ESCO1 silencing was investigated in prostate cancer cells. Cell viability and the ability to metastasize were significantly inhibited in vitro following silencing of ESCO1. Knockdown of ESCO1 induced G0/G1 phase arrest and promoted apoptosis in prostate cancer cells. Additionally, using a subcutaneous injection animal tumor model, significant inhibition of tumor growth was observed with in vivo ESCO1 knockdown.
The Akt signaling pathways serve essential roles in controlling cell survival, proliferation, and tumor progression, and are frequently activated during tumorigenesis. Activation of the Akt signaling cascades promotes cell survival by inhibiting apoptosis (22-24). In the present study, ESCO1 silencing decreased the proportion of cancer cells in the G1 phase and increased pre-G1 apoptotic cells. As demonstrated in the western blotting assay, phosphorylation of Akt was inhibited by ESCO1 knockdown. The downregulation of phosphorylated Akt suggests that the anti-tumor effect of ESCO1 silencing on prostate cancer may be partially mediated by its effects on the Akt signaling pathway. Further investigation is required to identify the mechanism underlying the tumor-promoting role of ESCO1.
Conclusions
The results of the present study demonstrate that ESCO1 expression is significantly increased in prostate cancer. A statistically significant increase of ESCO1 was associated with higher Gleason scores, late TNM stages, raised PSA levels, frequency of biochemical recurrence and lymph node metastasis, and predicted a poor prognosis in prostate cancer. In vitro, ESCO1 was associated with viability, migration, and invasion of prostate cancer cells, whereas, in vivo, ESCO1 silencing significantly inhibited the growth of prostate tumors. These results indicate that ESCO1 is a promising prognosis biomarker and target for the treatment of patients with prostate cancer.
Acknowledgments
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.05.34). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was performed under a protocol approved by the Ethics Committee of the Air Force General Hospital (Beijing, China) in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent was received from all patients. The animal experiments were performed in accordance with the institutional ethical requirements of the Air Force General Hospital and approved by the Ethics Committee of Air Force General Hospital.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel R, Ward E, Brawley O, et al. Cancer statistics: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin 2011;61:212-36. [Crossref] [PubMed]
- Hsing AW, Tsao L, Devesa SS. International trends and patterns of prostate cancer incidence and mortality. Int J Cancer 2000;85:60-7. [Crossref] [PubMed]
- Lu S, Goering M, Gard S, et al. Eco1 is important for DNA damage repair in S cerevisiae. Cell Cycle 2010;9:3315-27. [Crossref] [PubMed]
- Hou F, Zou H. Two human orthologues of Eco1/Ctf7 acetyltransferases are both required for proper sister-chromatid cohesion. Mol Biol Cell 2005;16:3908-18. [Crossref] [PubMed]
- Zhang J, Shi X, Li Y, et al. Acetylation of Smc3 by Eco1 is required for S phase sister chromatid cohesion in both human and yeast. Mol Cell 2008;31:143-51. [Crossref] [PubMed]
- Rolef Ben-Shahar T, Heeger S, Lehane C, et al. Eco1-dependent cohesin acetylation during establishment of sister chromatid cohesion. Science 2008;321:563-6. [Crossref] [PubMed]
- Michaelis C, Ciosk R, Nasmyth K. Cohesins chromosomal proteins that prevent premature separation of sister chromatids. Cell 1997;91:35-45. [Crossref] [PubMed]
- Barber TD, McManus K, Yuen KW, et al. Chromatid cohesion defects may underlie chromosome instability in human colorectal cancers. Proc Natl Acad Sci U S A 2008;105:3443-8. [Crossref] [PubMed]
- Unal E, Heidinger-Pauli JM, Koshland D. DNA double-strand breaks trigger genome-wide sister-chromatid cohesion through Eco1 (Ctf7). Science 2007;317:245-8. [Crossref] [PubMed]
- Price JC, Pollock LM, Rudd ML, et al. Sequencing of candidate chromosome instability genes in endometrial cancers reveals somatic mutations in ESCO1, CHTF18, and MRE11A. PLoS One 2013;8:e63313. [Crossref] [PubMed]
- Luedeke M, Linnert CM, Hofer MD, et al. Predisposition for TMPRSS2-ERG fusion in prostate cancer by variants in DNA repair genes. Cancer Epidemiol Biomarkers Prev 2009;18:3030-5. [Crossref] [PubMed]
- Koksal IT, Dirice E, Yasar D, et al. The assessment of PTEN tumor suppressor gene in combination with Gleason scoring and serum PSA to evaluate progression of prostate carcinoma. Urol Oncol 2004;22:307-12. [Crossref] [PubMed]
- Leidinger P, Hart M, Backes C, et al. Differential blood-based diagnosis between benign prostatic hyperplasia and prostate cancer: miRNA as source for biomarkers independent of PSA level, Gleason score, or TNM status. Tumour Biol 2016;37:10177-85. [Crossref] [PubMed]
- Cahill DP, Lengauer C, Yu J, et al. Mutations of mitotic checkpoint genes in human cancers. Nature 1998;392:300-3. [Crossref] [PubMed]
- Rajagopalan H, Jallepalli PV, Rago C, et al. Inactivation of hCDC4 can cause chromosomal instability. Nature 2004;428:77-81. [Crossref] [PubMed]
- Wang Z, Cummins JM, Shen D, et al. Three classes of genes mutated in colorectal cancers with chromosomal instability. Cancer Res 2004;64:2998-3001. [Crossref] [PubMed]
- Solomon DA, Kim T, Diaz-Martinez LA, et al. Mutational inactivation of STAG2 causes aneuploidy in human cancer. Science 2011;333:1039-43. [Crossref] [PubMed]
- Rocquain J, Gelsi-Boyer V, Adelaide J, et al. Alteration of cohesin genes in myeloid diseases. Am J Hematol 2010;85:717-9. [Crossref] [PubMed]
- Kattan MW, Eastham JA, Wheeler TM, et al. Counseling men with prostate cancer: a nomogram for predicting the presence of small, moderately differentiated, confined tumors. J Urol 2003;170:1792-7. [Crossref] [PubMed]
- Steyerberg EW, Roobol MJ, Kattan MW, et al. Prediction of indolent prostate cancer: validation and updating of a prognostic nomogram. J Urol 2007;177:107-12. [Crossref] [PubMed]
- Ecke TH, Schlechte HH, Hubsch A, et al. TP53 mutation in prostate needle biopsies--comparison with patients follow-up. Anticancer Res 2007;27:4143-8. [PubMed]
- McCubrey JA, Steelman LS, Abrams SL, et al. Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT pathways in malignant transformation and drug resistance. Adv Enzyme Regul 2006;46:249-79. [Crossref] [PubMed]
- Hammarsten P, Cipriano M, Josefsson A, et al. Phospho-Akt immunoreactivity in prostate cancer: relationship to disease severity and outcome, Ki67 and phosphorylated EGFR expression. PLoS One 2012;7:e47994. [Crossref] [PubMed]
- Brunet A, Bonni A, Zigmond MJ, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 1999;96:857-68. [Crossref] [PubMed]


