Chemoembolization with CalliSpheres drug-eluting beads loaded with irinotecan in the treatment of unresectable colorectal cancer liver metastases: preliminary results in 16 cases
Introduction
Colorectal cancer (CRC) is one of the most common malignant tumors, and CRC patients can easily develop intrahepatic metastasis. According to literature, liver metastases occur in up to 70% of CRC patients, 50% of whom eventually die (1). Although partial hepatectomy remains the first-line treatment for metastatic lesions confined to the liver, only 20–30% of these lesions are resectable (1) and 70–80% of patients will experience tumor recurrence within 5 years (2).
For patients with colorectal cancer liver metastasis (CRCLM) that cannot be surgically removed, intravenous chemotherapy is the first-line treatment. Both FOLFOX (leucovorin, 5-Fluorouracil, and oxaliplatin) and FOLFIRI (leucovorin, 5-Fluorouracil, and irinotecan) protocols have dramatically improved patients’ survival (3). Also, the rapid advances in targeted therapy further promote survival prolongation (3,4). The median survival of CRCLM patients has been extended to about 30 months (5).
Transcatheter arterial chemoembolization (TACE) including conventional TACE (cTACE) and drug-eluting beads transarterial chemoembolization TACE (DEB-TACE) are often used to treat primary and secondary tumors in the liver (6) by blocking blood supply to tumors and slowly release the chemotherapy drugs into the tumors (7). It was initially used in the treatment of unresectable primary liver cancer but nowadays is also applied in treating CRCLM. As novel embolic agents, drug-eluting beads (DEBs) can achieve permanent embolization and slow local release of drugs, showing remarkable advantages (8,9).
The most widely-used DEBs for the treatment of CRCLM is DC Bead (DC Bead Biocompatibles Ltd., UK). CalliSpheres drug-eluting beads was an independently developed product in China (Hengrui-Callisyn, Suzhou, China; National Machinery Registration No. 20153771072). Since it has been marketed not long before, few clinical studies have explored its application in the chemoembolization for CRCLM. By analyzing the clinical data of 16 patients with CRCLM who were treated with CalliSpheres DEBs loaded with irinotecan (DEBIRI) in our center from March 2017 to December 2018, we summarized the short-term efficacy and perioperative complications of this technology, with an attempt to further clarify its safety and effectiveness.
Methods
Subjects
In this retrospective, single-center, one-arm clinical study, we analyzed the clinical data of 16 patients with CRCLM who were treated with chemoembolization using DEBIRI in our center from March 2017 to December 2018. There were 10 males and 6 females aged 45–68 years (mean: 54.6 years). Table 1 summarizes the baseline data of these patients. All 16 patients had a history of surgical resection of the primary lesions. They were treated with DEBIRI because of the progression of intrahepatic lesions after chemotherapy or their reluctance to receive intravenous chemotherapy based on the will of the patients and/or their families. Of the 16 patients, 11 were wild type KRAS (68.7%), and 5 were mutant Kras (31.3%). Eleven patients underwent systemic therapy, of whom 11 patients completed the first-line regimen, 8 patients completed the second-line regimen; 3 patients were treated combined with cetuximab, and 6 patients was treated combined with bevacizumab. Intrahepatic lesions were confirmed by CT/MRI plain and enhanced scans. The inclusion and exclusion criteria were established according to literature (10,11). The inclusion criteria included: (I) the primary tumor had been resected, and the CRCLM was pathologically or radiologically confirmed; after at least 6 cycles of systemic chemotherapy with irinotecan- or oxaliplatin-based protocols, the intrahepatic lesion progressed, but there was no recurrence in the primary lesion; there was no metastasis to other organs; or, the patient refused to receive intravenous chemotherapy; (II) both male and female patients aged 18–70 years; (III) with an ECOG score of <2; (IV) with an estimated life expectancy of 3 months or more; (V) with lesions confined to the liver, and the level of hepatic parenchymal involvement did not exceed 60%; (VI) with Child-Pugh class A or B; and (VII) without a history of interventional therapy (e.g., TACE, cryoablation, microwave or radiofrequency ablation, I125 particle implantation, or intratumoral injection of anhydrous ethanol) for the intrahepatic lesions. The exclusion criteria included: (I) with the contraindications of TACE; (II) with severe or uncontrolled infections; (III) with severe heart disease; (VI) with severe or poorly-controlled diabetes and/or hypertension; (V) with infectious or neoplastic ascites; (VI) with other distant metastatic lesions outside the liver; (VII) with obvious an arteriovenous fistula that cannot be embolized, as revealed by angiography; (VIII) with severe bone marrow suppression after chemotherapy; (IX) unable to take care of themselves or cooperate during the interventional therapy; and (X) use of molecularly targeted drugs such as apatinib.
Table 1
| Parameters | Number (%) |
|---|---|
| Gender | |
| Male | 10 (62.5) |
| Female | 6 (37.5) |
| Age (years) | 54.6 (45 to 68) |
| Liver function (Child-Pugh class) | |
| A | 14 (87.5) |
| B | 2 (12.5) |
| ECOG score | |
| 0 | 14 (87.5) |
| 1 | 2 (12.5) |
| Primary tumor | |
| Colonic cancer | 10 (62.5) |
| Rectal cancer | 6 (37.5) |
| Intrahepatic lesions | |
| Single lobe | 5 (31.3) |
| Multiple lobes | 11 (68.7) |
| Liver involvement | |
| ≤30% | 4 (25.0) |
| ≤60% | 12 (75.0) |
| Previous chemotherapy protocol | |
| Refusing intravenous chemotherapy | 5 (31.3) |
| First line | 11 (68.7) |
| Second line | 8 (50.0) |
| Kras | |
| Wild type | 11 (68.7) |
| Mutated | 5 (31.3) |
| FOLFOX | 3 (18.8) |
| FOLFIRI | 8 (50.0) |
| Cetuximab | 3 (18.8) |
| Bevacizumab | 6 (37.5) |
Data are presented as mean ± SD, median (25th–75th) or count (%). ECOG, Eastern Cooperative Oncology Group; FOLFOX: folinic acid + fluorouracil + oxaliplatin; FOLFIRI: folinic acid +fluorouracil + irinotecan.
Routine examinations before chemoembolization procedure
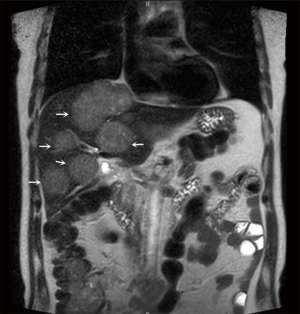
The patients underwent imaging and routine ECG examination, chest and abdominal CT/MR plain scans/contrast-enhanced examinations to identify the lesions confined to the liver, and to assess the size, number of lesions and the proportion of the liver invasion being invaded (Figure 1). Blood biochemical tests were performed for blood cell counts, liver and kidney functions, bleeding and clotting time, hepatitis B virus/hepatitis C virus, tumor marker [carcinoembryonic antigen (CEA)], and other indicators. Meanwhile, hepatoprotective symptomatic treatments were provided as appropriate. After the examination results became available, the liver function was assessed according to Child-Pugh classification. Morphine hydrochloride 10 mg was intra-muscularly injected for analgesia 30 minutes before the operation. The intravenous access was placed, and its patency was maintained with normal saline.
Chemoembolization with DEBIRI
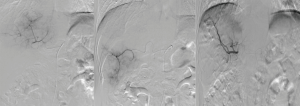
All procedures were performed under digital subtraction angiography (DSA) in the interventional operating room of our center. The chemoembolization was performed by a deputy chief physician or an attending physician with more than 10 years of experience under the guidance of a chief physician.
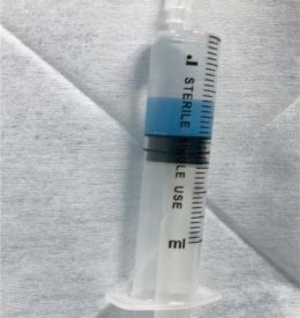
The procedural process was as follows: (I) disinfection and draping were routinely performed in the inguinal field. After femoral artery puncture under local anesthesia, the guide wire and catheter were inserted using the Seldinger technique; (II) after a Pig-tail angiographic catheter (Cook, USA) was placed for abdominal aortogram, a Yashiro catheter (Terumo, Japan) was introduced into the celiac artery and superior mesenteric artery for angiography, to identify the site, number, and feeding vessels of the tumors; (III) the DEBs were applied, during which one vial of CalliSpheres DEBs (1 g: 2 mL) with a particle size of 100–300 µm was loaded with one vial of irinotecan hydrochloride [100 mg/5 mL; Pfizer (Perth) Pty Limited, Australia]. More specifically, one vial of CalliSpheres DEBs was drawn with a 20-mL syringe and left at room temperature for 5 min before the supernatants was discarded. Then, 5 mL of liquid irinotecan hydrochloride was added and let stand for 30 min, during which the mixture was gently shaken every 5 minutes (Figure 2). After 30 minutes, 12 mL of non-ionic contrast agent was added, and the concentration of the mixture was adjusted with 3–5 mL of water for injection, so as to ensure the microspheres were naturally suspended in the liquid. Then, the syringe was connected to a T-tube and a 3-mL syringe; (IV) after super selective catheterization into the tumor feeding artery with a microcatheter (Progreat; Terumo, Tokyo, Japan), 2 mL of 1% lidocaine solution was slowly injected; (V) the microcatheter was connected into a T-tube. A 3-mL syringe was used to draw 3 mL of DEBs mixed with contrast agent, which was slowly injected into the microcatheter at a rate of 1 mL/min. Meanwhile, fluoroscopy was used to monitor the flow and distribution of the mixture of DEBs and contrast agent, during which any injury to gallbladder artery, right gastric artery, and non-targeted blood vessels should be avoided (Figure 3). The microcatheter was intermittently and slowly rinsed with normal saline; (VI) a subjective angiographic chemoembolization endpoint (SACE) level III (12) was selected as the chemoembolization endpoint (i.e., the tumor staining diminished and the tumor-feeding vessels decreased). Fade branch anatomy was observed during angiography; (VII) after reaching the embolization endpoint, a second angiography was performed after waiting for 5 minutes. If the embolization endpoint was found to be degraded, further embolization sessions were performed until the embolization endpoint reached stable status; (VIII) if the embolization end point was still not reached after one vial of DEBs was used up, the treatment would be stopped according to the protocol recommended in the literature (11), and a second treatment session would be initiated during the subsequent procedure; (IX) if an arteriovenous fistula was found in the tumor area during angiography, super selective microcatheterization would be performed. Large blank microspheres (500–700 µm) were used to embolize the fistula before DEBIRI treatment. If the fistula could not be handled, the patient would be ruled out from the DEBIRI treatment group.
Postprocedural management
After the patient returned to the ward, symptomatic supports were offered for liver protection, analgesia, and to stop vomiting. Changes in vital signs were monitored. For elderly patients, special attention should be paid to their blood glucose and blood pressure. Changes in the pulsation of the dorsal artery of the right lower extremity and the skin color should also be observed.
Follow-up plan and subsequent treatment
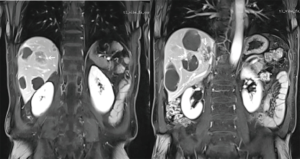
Patients were asked to return for a follow-up visit 3–4 weeks after each DEBIRI procedure. The follow-up evaluation included upper abdominal CT/MRI plain and contrast-enhanced scans (Figure 4), measurements of tumor markers (e.g., CEA) and liver and kidney function tests. Based on the evaluation results, patients received further treatment or were arranged to receive the next follow-up visits. According to the recommended protocol in the literature (11) and the distribution, size, number, and staining results of the lesion, the treatment plan and sessions were designed: the lesions within a single lobe were treated with up to 2 DEBIRI sessions; the multi-lobar lesions were treated with up to 4 DEBIRI sessions performed within one lobe treatments. The intervals between two sessions were 3–4 weeks and the Child-Pugh class of liver function could reach the baseline level before treatment.
Evaluation methods
Successful operation
During successful embolization sessions, the catheter successfully accesses the target vessel and delivers DEBs to the target area, reaching the expected endpoint of embolization. No procedure-related death occurs during the hospitalization.
Response evaluation
Response evaluation was performed according to the Modified Response Evaluation Criteria in Solid Tumors (mRECIST) (13). Complete response (CR) was defined as the disappearance of any intratumoral enhancement at CT. Partial response (PR) was defined as at least a 30% decrease in the sum of the diameters of the visible target lesions compared to the baseline measurements. Stable disease (SD) was defined as any case that did not meet the definition of either PR or progressive disease. Progressive disease (PD) was defined as at least a 20% increase in the sum of the diameters of the visible target lesions compared to the smallest measurements recorded since the start of the treatment.
Objective response rate (ORR)
ORR was defined as the proportion of CR + PR cases out of the population of patients with measurable disease at baseline.
Disease control rate (DCR)
DCR was defined as the proportion of CR + PR + SD cases out of the population of patients with measurable disease at baseline.
Safety assessment
All the complications were classified into 2 categories according to the literature (14). Adverse events (AE) were categorized according to the clinical practice guidelines of the Society of Interventional Radiology. Major AEs included those needed an increased level of care, major therapy, prolonged hospitalization, and those within permanent sequelae or death. Minor AEs were defined as those required only nominal therapy or observation. The severity of the recorded AEs was graded.
Cancer Therapy Evaluation Program, Common Terminology Criteria for Adverse Events, Version 3.0 was used to report clinical adverse events (15). Grade 0: no AE or within normal limits; grade 1: mild AE with marginal clinical relevance; grade 2: moderate AE requiring minimal/local/noninvasive intervention; grade 3: severe undesirable AE requiring hospitalization/ invasive intervention/transfusion/operation; grade 4: life-threatening or disabling AE; and grade 5: fatal AE. Changes in blood cells (WBC and PLT), liver function (ALB, ALT, AST, TBIL, and DBIL), and tumor marker (CEA) were recorded before and during follow-up visits.
Statistical analysis
Statistical analysis was performed by using the Stata 12.0 software. Measurement data were expressed as mean ± standard deviation. The changes in tumor marker CEA, liver function, and blood cells before and after treatment were analyzed by a paired-sample t-test. P value of <0.05 was considered statistically significant, and P value of <0.01 was considered highly statistically significant.
Results
Treatment
A total of 46 DEBIRI sessions were performed in 16 patients (2 sessions in 5 cases, 3 sessions in 8 cases, and 4 sessions in 3 cases; mean: 2.8 sessions). The success rate was 100.0%. No patient died during the hospitalization. The average length of hospital stay generally, and the length after DEBIRI was 10.2 days (range, 7–15 days) and 7 days (range, 5–10 days). All patients were followed up for 6–14 months (mean: 8 months).
Postprocedural complications
After 46 DEBIRI sessions, the major AEs included acute cholecystitis (2.2%), right upper quadrant pain (76.1%), nausea (89.1%), vomiting (84.8%), and hypertension (87.0%). In one patient with acute cholecystitis, symptomatic treatments for protecting liver and gallbladder and preventing infections were applied for 5 days, and then the abdominal pain was relieved. Other complications were resolved 3–5 days after symptomatic supports including analgesia and to stop vomiting. The minor AEs were mainly associated with chemotherapy drugs or post-embolization syndrome. They were mild or moderate, mainly in grades 1–2 (Table 2).
Table 2
| Type | Number (%) | Grade |
|---|---|---|
| Acute cholecystitis | 1 (2.2) | 3 |
| Hypertension | 40 (87.0) | 1–2 |
| Infections | 2 (4.3) | 1,2 |
| Nausea | 41 (89.1) | 1–2 |
| Vomiting | 39 (84.8) | 1–2 |
| Right upper quadrant pain | 35 (76.1) | 1–2 |
| Fever | 10 (21.7) | 1 |
| Diarrhea | 3 (6.5) | 1 |
| Fatigue | 12 (26.1) | 1 |
DEBIRI, drug-eluting beads loaded with irinotecan.
Response evaluation
All 16 patients completed the treatment plan and were followed up regularly. No patient died during the follow-up period. The treatment response was evaluated by using m-RECIST 3 and 6 months after the initiation of DEBIRI treatment. The 3- and 6-month CR, PR, SD, PD, ORR, and DCR were 0.0%, 68.7%, 31.3%, 0.0%, 68.7%, and 100.0% vs. 0.0%, 81.2%, 12.5%, 6.2%, 81.2%, and 93.7% (Table 3). Changes in the test results and CEA before treatment and during follow-up are shown in Table 4. The median of CEA significantly decreased by 47% after treatment (P<0.01). Other indicators including AST, ALT, ALB, TBIL, DBIL, WBC, and PLT showed no significant difference (P>0.05).
Table 3
| Parameters | After 3 months, n (%) | After 6 months, n (%) |
|---|---|---|
| CR | 0 (0.0) | 0 (0.0) |
| PR* | 11 (68.7) | 13 (81.2) |
| >90% | 2 (12.5) | 4 (25.0) |
| 60–90% | 4 (25.0) | 6 (37.5) |
| 30–60% | 5 (31.3) | 3 (18.8) |
| SD | 5 (31.3) | 2 (12.5) |
| PD | 0 (0.0) | 1 (6.2) |
| ORR (CR + PR) | 11 (68.7) | 13 (81.2) |
| DCR (CR + PR + SD) | 16 (100.0) | 15 (93.7) |
*, When contrast-enhanced CT/MRI scans were performed, the enhanced tumor areas were shrunken by >90%, 60–90%, and 30–60% (when compared with the baseline data), which are divided into three categories and their numbers and percentages are calculated respectively. mRECIST, modified Response Evaluation Criteria in Solid Tumors; DEBIRI, drug-eluting beads loaded with irinotecan.
Table 4
| Parameter | ALT (U/L) | AST (U/L) | ALB (g/L) | TBIL (µmol/L) | DBIL (µmol/L) | WBC (×109/L) | PLT (×1012/L) | CEA (µg/L) |
|---|---|---|---|---|---|---|---|---|
| Before chemoembolization | 33.68±20.53 | 31.63±12.61 | 34.45±16.12 | 12.73±5.46 | 10.61±5.79 | 6.39±3.36 | 173.30±128.20 | 194.92±58.80 |
| During follow-up | 35.35±19.72 | 35.28±28.59 | 41.63±8.75 | 15.91±6.56 | 12.37±5.53 | 5.27±2.74 | 168.37±146.28 | 104.15±77.60 |
| t | 0.2345 | 0.4627 | 1.5658 | 1.4903 | 0.8793 | 1.0330 | 0.1014 | 3.2848 |
| P | 0.8161 | 0.6437 | 0.1279 | 0.1466 | 0.3862 | 0.3097 | 0.9199 | 0.0026 |
Discussion
CRC ranks first among the gastrointestinal tumors in the annual incidence and is the third most common malignant tumor in Europe and North America. In China, it ranks second place among gastrointestinal tumors and the fourth place among all tumors. In recent years, its incidence has dramatically increased (16). The liver is the most important target organ for CRC, and epidemiological data have confirmed that about 25% of CRC patients have liver metastases at diagnosis and 15–25% of patients may experience liver metastasis after radical resection of the primary lesions. The vast majority (80–90%) of CRCLM cannot be radically resected (17).
Intravenous chemotherapy remains the first-line treatment for unresectable CRCLM. In the first- and second-line systemic therapy, chemotherapeutic drugs included 5-fluorouracil and leucovorin combined with oxaliplatin or irinotecan. The patients were treated with systemic therapy plus biological therapy according to their status of Kras status. If for Kras wild type, chemotherapeutic drugs combined with cetuximab was recommended, for such patients, EGFR inhibitors can be used for 3-line therapeutic regimens. For KRAS mutation type, it was suggested bevacizumab should be included in the 1/2-line chemotherapy regimen (5,18).
It is believed that most CRCLMs are confined to the liver for a long period without extrahepatic spread and therefore local treatment is clinically valuable for CRCLM (19). A variety of local treatments including cryoablation, microwave ablation, radiofrequency ablation, I125 implantation, local injection of absolute ethanol, and cTACE have been introduced. Among them, cTACE has long been adopted and can be performed in many medical centers. However, due to the poorly standardized chemotherapy drugs and unstable clinical operations, the treatment guidelines of the National Comprehensive Cancer Network (NCCN) version 1.2013 considers cTACE a Category 3 recommendation based on insufficient data and variations in techniques among institutions (20).
After its first application in clinical practice in 2006, DEBs have remarkably increased tumor response rate and lowered drug toxicity when compared with cTACE (21). Clinical studies on DEB-TACE with DEBIRI for CRCLM have been carried out in developed countries, with remarkable achievements. In a multi-center RCT, Fiorentini et al. compared DEBIRI versus FOLFIRI in a head-to-head design and found that overall survival (OS) (22 vs. 15 months), progression-free survival (PFS) (7 vs. 4 months), and duration of extrahepatic metastasis (13 vs. 9 months) were significantly different between these two groups. The toxic effects of the chemotherapy drugs were significantly reduced, and the duration of improved quality of life was significantly prolonged (22). DEBIRI treatment is still an active and effective method for patients who are resistant to traditional chemotherapy (23,24). Martin II et al. had attempted to combine DEBIRI with FOLFOX and bevacizumab to treat metastatic colorectal cancer (mCRC) confined to the liver (25). The treatment guidelines of the NCCN v. 3.2013 advises that DEBIRI should be considered selectively and only at institutions with experience. No category recommendations are reported (20). According to Fiorentini, it represents the “regeneration” of chemoembolization in the treatment of CRCLM (21).
CalliSpheres DEBs are new embolic agents developed independently by a Chinese manufacturer and has been granted with an independent intellectual property right and a national invention patent. This product has a regular shape, uniform particle size, smooth surface, and good suspension performance.
Also, it is featured by variable elasticity, good compressibility, fast drug-loading speed, and long-lasting release time. The material of this product is similar to the DC Bead material, and the drug-loading mechanism and the sustained-release effect are the same (26). Since this product has just been marketed, few clinical studies have investigated it. No literature has described the application of DEBIRI for treating CRCLM. Analysis of the clinical data in our current series showed that the use of CalliSpheres in DEBIRI treatment had little impact on the key indicators of liver function and routine blood tests, and no bone marrow suppression or obviously-impaired liver function was observed. Compared with the data reported by Ranieri et al. (27), the systemic side effects rate of our group was higher, such as abdominal pain (21% vs. 76%), hypertension (30% vs. 87.0%), vomiting (7% vs. 84.8%), fever (16% vs. 21.7%), diarrhea (2% vs. 6.5%). However, the average grade of complications was about 1–2, which was consistent with relative documents reported. No liver abscess was found in this group. The factors for higher clinical complications may be explained as followed: the use of different DEBs; the technical operation was not standardized enough, and the recording methods for clinical complications were inconsistent and so on.
The main complications after the chemoembolization included post-embolization syndrome (composed of right upper quadrant pain and nausea/vomiting) and chemotherapy-associated toxicities. Abdominal pain could occur during the procedure, but its incidence and severity were significantly higher than those during DEB-TACE treating hepatocellular carcinoma. Therefore, active intervention should be offered to ensure a successful procedure. Postprocedural analgesia after returning to the ward was performed predominantly with moderate analgesic drugs such as tramadol, and potent analgesics such as morphine hydrochloride were rarely used. The incidence of hypertension (mainly grade 1 or 2) was high, which might be associated with post-embolization pain. Therefore, analgesics and oral antihypertensive drugs were applied. Typically, the hypertension was alleviated with the control of abdominal pain. Although the incidences of nausea/vomiting and other complications were high, these symptoms were mild and could be effectively alleviated after routine treatment. Acute cholecystitis caused by embolization of the cystic artery was a relatively severe event. One of our patients suffered from acute cholecystitis after the treatment. Retrospective analysis of his angiographic data revealed that the catheter tip had advanced beyond the gallbladder artery-opening; however, when angiography was performed upon the completion of embolization, the excessive dose of contrast agent caused the DEBs reflux, leading to the embolization of the gallbladder artery. Therefore, the location of the catheter tip, the setting of the high-pressure syringe and the total amount of contrast agent should be reasonably adjusted during postoperative angiography. Due to the newly developed project, the team was very cautious. And it was very strict about selecting patients in regard to their ECOG and liver function Child-Pugh grading. Therefore, the data of the patients’ overall length of hospital stay is better than that reported (27).
The proper positioning of the microcatheter tip remains controversial during super-selective catheterization. The European recommended protocol emphasizes the value of “lobar approach” (11): the tip of the microcatheter is maintained at the hepatic artery level, and any injury to the gallbladder artery and right gastric artery should be avoided, and the DEBs should be slowly injected to achieve chemoembolization at the lobar level. The rationales of this approach include: (I) clinically invisible micrometastases may be missed during super-selective embolization; (II) irinotecan must be converted by liver CES-1/CES-2 enzymes to the active metabolite SN-38 before exerting its biological effects; (III) more drugs embedded in the normal liver parenchyma may contribute to the generation of SN38 and then exert a cytotoxic effect; and (IV) the medication is more important than embolization, and its purpose is to treat all metastases of the whole liver, including potential lesions not detected by imaging studies. Among our patients who received early treatment, follow-up imaging showed that the necrosis at the center of the tumor was complete, but there were ring-like residues at the periphery of the tumors. After it was confirmed that there were no extrahepatic feeding vessels, the lobar approach was applied to guide the positioning of the microcatheter tip. Further efficacy was achieved after a second treatment session. Analysis of CRCLM revealed that the blood supply of tumors was low or moderate, and the feeding arteries were not remarkably thickened; staining showed there was no definite border between tumor and its neighboring hepatic tissues, and super selective cauterization might miss the tumor-feeding arteries.
Analysis of the short-term treatment response in our current study showed that the 3- and 6-month CR, PR, and ORR were 0.0%, 68.7%, 68.7% and 0.0%, 81.2%, 81.2%, respectively. The possible reason for such differences was that some of the multinodular non-single-lobe lesions were identified at the 3-month follow-up visit, during which the treatment plan had not been completed; after the treatment plan was completed, the treatment response was improved in 2 patients (as “SD” at the 3-month follow-up visit). A further grouping of PR patients according to the proportion of enhanced tumor shrinkage under CT/MRI contrast-enhanced scans showed that the degree of tumor necrosis increased at the 6-month follow-up visit. A new lesion was found in one patient who had achieved SD at the 3-month follow-up visit and was identified as PD during the follow-up. Ranieri et al. has ever reported that DEBIRI with HepaSphere beads was once used to treat CRCLM (27). The response was as followed: CR 21.8%, PR 13%, SD 52.2%, PD 13%, RR 34.8%. Compared with the results of this group, we can see that the CR in our group was lower (0%) and RR was higher (68.7%). The differences between them in data may be attributed to the following factors: the small number of samples in this study; the different number of DEBIRI treatment times; the different size of tumors; the different number of patients with unilobe/multilobe tumors; the different dosage of chemotherapeutic drugs and so on.
Research on the particle sizes for DEBs has suggested that small particle sizes not only achieve better therapeutic results but also have fewer complications (28). A variety of particle sizes including 500–700, 300–500, and 100–300 µm have been applied in clinical studies, which might have affected the efficacies (22). In our current study, the recommended protocol in technical guidelines (11) was applied, and the particle size used was 100–300 µm, which achieved a satisfactory therapeutic response.
Our study had some limitations. First, due to its small sample size, it is impossible to carry out a more detailed stratification. Second, we first described the treatment of CRCLM with CalliSpheres loaded with irinotecan, focusing on perioperative safety and short-term efficacy; however, since overall survival (OS) remains the gold standard for evaluating the efficacy of a treatment strategy, the sample size and follow-up duration need to be increased. Third, although the short-term efficacy data of our current study was similar to those reported in the literature, more multi-center, large-sample, prospective and controlled trials are required to further demonstrate the difference, if any, between CalliSpheres and DC Bead. Fourth, treatment of CRCLM with DEBIRI has been widely applied in western countries, and relevant technical recommendations have been available. As a newly-introduced technique in China, DEBIRI requires further learning and practice, especially in the management of its complications.
Conclusions
CalliSpheres DEBs is a safe treatment strategy for CRCLM, with a high local response rate. Future research directions may include smaller particle size of DEBs; loading of more chemotherapeutic drugs or targeted drugs; appropriate timing of DEBs intervention in the multidisciplinary treatment of CRCLM; and the combination of DEBs with intravenous chemotherapy. The consequence of hypoxia caused by embolization is the adaptive response of increasing production of vascular endothelial growth factor (VEGF) that stimulates angiogenesis and cause tumor recurrence. Therefore, the study of DEBIRI combined with angiogenesis inhibitors may become a research hotspot (29).
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.05.14). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Shenzhen People’s Hospital (IRB number: LL-KT-201703008) and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Young S, D’Souza D, Flanagan S, et al. Review of the Clinical Evidence for the Use of DEBIRI in the Treatment of Colorectal Metastatic Disease. Cardiovasc Intervent Radiol 2017;40:496-501. [Crossref] [PubMed]
- Fong Y. Hepatic colorectal metastasis: current surgical therapy, selection criteria for hepatectomy, and role for adjuvant therapy. Adv Surg 2000;34:351-81. [PubMed]
- Sinicrope FA, Okamoto K, Kasi PM, et al. Molecular biomarkers in the personalized treatment of colorectal cancer. Clin Gastroenterol Hepatol 2016;14:651-8. [Crossref] [PubMed]
- Ohhara Y, Fukuda N, Takeuchi S, et al. Role of targeted therapy in metastatic colorectal cancer. World J Gastrointest Oncol 2016;8:642-55. [Crossref] [PubMed]
- Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 2016;27:1386-422. [Crossref] [PubMed]
- Liapi E, Geschwind J. Chemoembolization for primary and metastatic liver cancer. Cancer J 2010;16:156-62. [Crossref] [PubMed]
- Aliberti C, Tilli M, Benea G, et al. Trans-arterial chemoembolization (TACE) of liver metastases from colorectal cancer using irinotecan-eluting beads: preliminary results. Anticancer Res 2006;26:3793-5. [PubMed]
- Lewis AL, Gonzalez MV, Lloyd AW, et al. DC Bead: in vitro characterization of a drug-delivery device for transarterial chemoembolization. J Vasc Interv Radiol 2006;17:335-42. [Crossref] [PubMed]
- Taylor RR, Tang YQ, Gonzalez MV, et al. Irinotecan drug eluting beads for use in chemoembolization: in vitro and in vivo evaluation of drug release properties. Eur J Pharm Sci 2007;30:7-14. [Crossref] [PubMed]
- Martin RCG, Joshi J, Robbins K, et al. Transarterial Chemoembolization of Metastatic Colorectal Carcinoma with Drug-Eluting Beads, Irinotecan (DEBIRI): Multi-Institutional Registry. J Oncol 2009; [Crossref] [PubMed]
- Lencioni R, Aliberti C, Baere TD, et al. Transarterial Treatment of Colorectal Cancer Liver Metastases with Irinotecan-Loaded Drug-Eluting Beads: Technical Recommendations. J Vasc Interv Radiol 2014;25:365-9. [Crossref] [PubMed]
- Jin B, Wang DX, Lewandowski RJ, et al. The Impact of Chemoembolization Endpoints on Survival in Hepatocellular Carcinoma Patients. AJR Am J Roentgenol 2011;196:919-28. [Crossref] [PubMed]
- Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010;30:52-60. [Crossref] [PubMed]
- Cardella JF, Kundu S, Miller DL, et al. Society of Interventional Radiology. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol 2009;20:S189-91. [Crossref] [PubMed]
- Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: Development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 2003;13:176-81. [Crossref] [PubMed]
- Coimbra FJ, Pires TC, Costa JWL, et al. Advances in surgical treatment of colorectal liver metastases. Rev Assoc Med Bras 2011;57:220-7. [Crossref] [PubMed]
- Manfredi S, Lepage C, Hatem C, et al. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg 2006;244:254-9. [Crossref] [PubMed]
- Yoshino T, Arnold D, Taniguchi H, et al. Pan-Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: a JSMO-ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Ann Oncol 2018;29:44-70. [Crossref] [PubMed]
- Aliberti C, Fiorentini G, Muzzio PC, et al. Trans-arterial chemoembolization of metastatic colorectal carcinoma to the liver adopting DC Bead®, drug-eluting bead loaded with irinotecan: results of a phase II clinical study. Anticancer Res 2011;31:4581-7. [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology - Colon Carcinoma, version 1. Fort Washington, PA: NCCN, 2013.
- Fiorentini G, Aliberti C, Mulazzani L, et al. Chemoembolization in Colorectal Liver Metastases: The Rebirth. Anticancer Res 2014;34:575-84. [PubMed]
- Fiorentini G, Aliberti C, Tilli M, et al. Intra-arterial Infusion of Irinotecan-loaded Drug-eluting Beads (DEBIRI) versus Intravenous Therapy (FOLFIRI) for Hepatic Metastases from Colorectal Cancer: Final Results of a Phase III Study. Anticancer Res 2012;32:1387-95. [PubMed]
- Bhutiani N, Akinwande O, Martin RCG. Efficacy and toxicity of hepatic intraarterial Drug-Eluting (Irinotecan) Bead (DEBIRI) therapy in Irinotecan-Refractory unresectable colorectal liver metastases. World J Surg 2016;40:1178-90. [Crossref] [PubMed]
- Martin RCG, Joshi J, Robbins K, et al. Hepatic Intra -Arterial injection of Drug-Eluting Bead, Irinotecan (DEBIRI) in unresectable colorectal liver metastases refractory to systemic chemotherapy: Results of multi-institutional study. Ann Surg Oncol 2011;18:192-8. [Crossref] [PubMed]
- Martin RCG II, Scoggins CR, Schreeder M, et al. Randomized Controlled Trial of Irinotecan Drug-Eluting Beads With Simultaneous FOLFOX and Bevacizumab for Patients With Unresectable Colorectal Liver-Limited Metastasis. Cancer 2015;121:3649-58. [Crossref] [PubMed]
- Zhang S, Huang C, Li Z, et al. Comparison of pharmacokinetics and drug release in tissues after transarterial chemoembolization with doxorubicin using diverse lipiodol emulsions and CalliSpheres Beads in rabbit livers. Drug Deliv 2017;24:1011-7. [Crossref] [PubMed]
- Ranieri G, Asabella M, Fazio AV, et al. A pilot study employing hepatic intra-arterial irinotecan injection of drug-eluting beads as salvage therapy in liver metastatic colorectal cancer patients without extrahepatic involvement: the first southern Italy experience. Onco Targets Ther 2016;9:7527-35. [Crossref] [PubMed]
- Akinwande OK, Philips P, Duras P, et al. Small Versus Large-Sized Drug-Eluting Beads (DEBIRI) for the Treatment of Hepatic Colorectal Metastases: A Propensity Score Matching Analysis. Cardiovasc Intervent Radiol 2015;38:361-71. [Crossref] [PubMed]
- Gadaleta CD, Ranieri G. Trans-arterial chemoembolization as a therapy for liver tumours: New clinical developments and suggestions for combination with angiogenesis inhibitors. Crit Rev Oncol Hematol 2011;80:40-53. [Crossref] [PubMed]