Stereotactic radiosurgery for brain metastases
Background
An estimated 1,665,540 Americans will be diagnosed with cancer in 2014 (1). Autopsy studies or surveys (2-4) from decades ago reported 10-50% of cancer patients developed brain metastases, however these studies were limited by methodological uncertainties. More recent large clinical studies (5,6) report around 9% incident proportions, suggesting more than 150,000 brain metastases are diagnosed in the United States each year. New systemic therapies may be increasing this incidence (7). Cerebral metastases are found most commonly at the junction of gray-white matter, where decreased caliber of blood vessels act as a trap for emboli. They are also more common at terminal watershed areas of arterial circulation. Patients present with a solitary metastasis in 30-50% of cases (8,9).
Clinical presentation
The development of brain metastases is a serious complication of progression of malignancy. Because the cranium is a rigid compartment, an enlarging mass can have a deleterious effect on neurologic function and duration of life. Twenty percent of brain metastases are diagnosed synchronously with the primary diagnosis, while 80% present later (2) (metachronous presentations). The most common symptoms associated with brain metastases are headache, mental status changes, focal weakness, seizure, and gait ataxia (10).
Diagnosis
The best imaging study for brain metastases is the contrast-enhanced MR, which is more sensitive and specific than CT. The MRI images the brainstem, temporal lobes & cerebellum better than CT, and T2-weighted sequence can detect even slight edema (11). Even MR is not very sensitive in detecting leptomeningeal disease, thus examination of CSF from one or more lumbar punctures is necessary if this presentation is suspected. If the clinical presentation is typical, and multiple lesions are detected, then there is little doubt as to the diagnosis. The clinician should to mindful to distinguish brain metastases from primary brain tumors, abscesses & other infections, hemorrhage, and infarction. Radiographic features favoring a diagnosis of a metastasis include smooth margins, location at gray-white junction, large amount of vasogenic edema despite small lesion, and the presence of multiple lesions (12). In a patient with a solitary brain mass, it may be important to confirm a diagnosis of metastatic disease, as management and prognosis may be influenced. The false positive rate for the MR diagnosis of single brain metastases has been reported (13) as high as 11%. Patients presenting with brain metastases without prior history of cancer will prove to have a lung primary 60% of the time.
Prognosis and patient selection
To decide on appropriate therapy for brain metastases, it is important to assess overall disease process and prognosis. One should consider the patient’s overall performance status, neurologic condition, and extent of systemic disease. Often patients have considerable systemic disease burdens which adversely affects prognosis. An assessment of prognosis can help determine how aggressively to treat intracranial disease. In 1997 the RTOG published (14) a method of categorizing patients with brain into prognostic groups. They evaluated outcomes in 1,200 patients treated on RTOG trials, performing recursive partition analysis (RPA) to determine which variables were predictive of prognosis. The most important prognostic factor was functional independence (Karnofsky Performance Status, or KPS ≥70). Patients with a KPS <70 had a median life expectancy of 2.3 months. Other important factors were uncontrolled versus controlled disease elsewhere, and age <65 years. Patients in RPA class one had KPS ≥70, primary disease controlled, and were <65 years old. In this group median survival was 7.1 months. RTOG RPA classification has been used in subsequent studies to stratify for prognosis and develop treatment recommendations.
Management
Treatment of brain metastases consists of corticosteroids, radiotherapy, surgical resection, radiosurgery, and chemotherapy.
Medical therapy
Symptomatic patients diagnosed with brain metastases should be started on steroids; 70% will respond (15). Patients with small, asymptomatic lesions do not require steroids, although administration may reduce effects of cranial RT. Dexamethaxone is the preferred corticosteroid due to a minimal mineralocorticoid affect, and low incidence of psychosis (16). The usual dose is 4 mg QID. Steroid should be tapered once symptoms resolve and after definitive therapy has been delivered. The median survival in patients treated with steroids alone is about 2 months (17,18). Brain metastases cause seizures in about 25% of pts. Prophylactic anticonvulsants do not reduce the incidence of new seizure in patients with newly diagnosed brain metastases (19), and should be reserved for patients who have had seizures. New chemotherapies have improved cancer outcomes, however most of these agents are ineffective against brain metastases.
Surgery
Surgical resection of a suspected brain metastasis may be appropriate to establish a tissue diagnosis, or in select patients to relieve symptomatic mass effect or edema. Another indication for resection is in functionally independent (KPS 70 or more) patients with solitary brain metastases: two prospective randomized trials (13,20) have demonstrated surgical resection plus WBRT improved overall survival compared to WBRT alone. In one of these trials, the benefit was limited to patients without active extracranial disease. Surgical resection of multiple lesions remains controversial, and is generally avoided.
Whole brain radiotherapy (WBRT)
WBRT is used for two purposes: to treat grossly evident brain metastases, and to address potential subclinical disease in patients at high risk for subsequent intracranial failure. Regarding the former indication, patients with brain metastases who receive supportive care alone have a median survival of only 51 days (17). WBRT has been a standard therapy for these patients, however this treatment yields median survival of only 3-6 months (21-23). A large prospective study showed WBRT resulted in a 38% radiographic response (24), while neurologic death occurs in up to 50% of brain metastases patients treated with WBRT (25). RTOG 9508 demonstrated local progression in about a third of patients one year following WBRT (26). The QUARTZ trial (27) which randomized WBRT against best supportive care failed to show WBRT improved survival; however the study was underpowered due to poor accrual. Thus while WBRT has been standard palliative therapy for intracranial metastases, rates and duration of response are suboptimal.
WBRT can eradicate subclinical intracranial disease, either in the resection cavity following surgical resection, or prophylactically elsewhere in the brain. In a randomized study (28), Patchell demonstrated that adding WBRT after complete surgical resection of solitary brain metastases reduced 1-year intracranial recurrence rates from 70% to 18%. The majority of the reduction in intracranial failures was at the resection cavity: local recurrence was reduced from 46% to 10%. WBRT reduced elsewhere brain recurrences from 37% to 14%. In this study WBRT reduced the risk for neurologic death, but did not impact overall survival or performance status.
Protracted courses of WBRT often interfere with systemic therapies. WBRT negatively impacts health-related quality of life (QOL) due to fatigue, hair loss and other side effects (29). WBRT detrimentally effects verbal memory function (30), particularly in patients with good performance status at baseline. A randomized trial (31) demonstrated that 52% of patients had significant reductions in learning and memory function 4 months following WBRT and SRS, versus only 24% when WBRT was omitted. Radiation-induced dementia and other serious neurologic sequelae have been reported in patients surviving 6 or more years after fractionated brain RT (32). These adverse effects, and the suboptimal efficacy of WBRT described above, have led investigators to pursue alternative focal therapy.
Stereotactic radiosurgery (SRS)
SRS is the precise delivery of focal radiotherapy to targets within the brain, employing multiple converging narrow radiotherapy beams. SRS is delivered using dedicated radiosurgery platforms (such as the GammaKnife or CyberKnife), or with modified medical linear accelerators (LINAC). Because the radiation beams approach from three dimensions, outside of the target the radiation dose falls off rapidly, i.e., at approximately the square of the distance from the target center. The dose fall-off is particularly steep for smaller tumors. The dose fall-off around tumors with larger diameters is less rapid, which limits the maximum safe prescription dose in larger tumors. Single fraction SRS is practical in patients without leptomeningeal involvement, and with lesions up to about 4 cm in maximum dimension. Outcomes following SRS alone vary considerably depending on the study: 1-year local control rates range from 70-95% (33-35), and 2-5-year local control rates fall to 50-70%. In a prospective randomized trial (36), SRS alone yielded local control rates of 72.5% at 1 year, falling to 48% at 2 years. Difference in cancer control are a function of tumor histology, size, and dose delivered, with better local control reported (37) in tumors less than 2 cm, and when marginal SRS dose exceeded 16 Gy.
SRS versus surgery
For numerous reasons, SRS is an attractive alternative to surgery for brain metastases. Compared to surgery, SRS eliminates the need for hospitalization, anesthesia and craniotomy, has a lower incidence of complications, and has a shorter recovery time. Unlike surgery, SRS can address multiple lesions anywhere in the brain. Post-operative RT to the surgical bed (or to the whole brain) is mandatory following surgery, but not required following SRS. Several retrospective comparisons (38-40) have suggested SRS yields similar outcomes to surgical resection of brain metastases. The only randomized trial (41) comparing SRS against surgery (plus WBRT) in patients with solitary brain metastases found no difference in survival or neurologic death; SRS had fewer grade 1-2 toxicities. While surgery is the preferred management of brain metastasis when a tissue diagnosis is required, when decompression is required to relieve mass effect or surrounded edema, and for solitary metastases larger than 3-4 cm in diameter, SRS is an appropriate alternative to surgery in other situations.
1-3 metastases treated with SRS
Multiple studies (42,43) have established the efficacy of SRS for solitary or multiple brain metastasis. RTOG 9508 (26) randomized patients with 1-3 brain metastasis to WBRT with or without SRS. The addition of SRS afforded a survival advantage in patients with solitary metastases, and in RPA class 1 patients. The improvement in survival for solitary metastases is analogous to that seen with surgical resection of solitary metastases (13). This study also showed that, in KPS greater than or equal to 70 patients with 2-3 metastases, adding a SRS boost to WBRT improved subsequent performance status. Thus all KPS ≥70 patients with solitary brain metastases should have either surgical resection or SRS. Patients with 2-3 metastases in RPA class 1 (KPS ≥70, disease elsewhere controlled and <65 years old) should also undergo SRS. And SRS should be considered other patients with 2-3 metastases. While all patients in RTOG 9508 received WBRT, other studies support approaching these patients with SRS alone.
Adding WBRT to SRS
Adding WBRT to SRS offers two potential advantages: addressing possible microscopic disease that may reside elsewhere in the brain, and increasing the total dose to the tumor, thereby improving local control rates. Regarding the latter, one retrospective analysis (44) showed adding WBRT to SRS improved local tumor control from 66% to 87%; another study from UCSF (45) showed adding WBRT to SRS did not affect local control. Both studies showed no impact on survival. A retrospective study from the University of Pittsburgh (37) showed adding WBRT to SRS improved local control only in patient with tumors larger than 2 cm, and when SRS dose was less than or equal to 16 Gy. In a prospective trial (36) which randomized patients with 1-4 metastases between SRS and SRS plus WBRT, Aoyama demonstrated that adding WBRT improved 1-year local control rates from 72.5% to 89%; at two years, adding WBRT to SRS improved local control from 48% to 89%. There was no difference in survival, however. Thus it appears that adding WBRT to SRS increases local control, particularly in larger tumors where lower SRS doses are delivered. This improvement in intracranial control does not translate into a survival advantage.
Since SRS only targets clinically evident sites of metastatic disease, the surrounding normal brain does not receive significant doses of radiotherapy. Thus SRS does not prophylactically treat potential microscopic metastases elsewhere in the brain, as does WBRT. However, the sparing of normal brain tissue from high radiation dose eliminates the cognitive deficits which can result from WBRT. In Aoyama’s randomized study (36) the addition of WBRT to SRS reduced rates of new brain metastases from 64% to 42% one year following treatment. Adding WBRT to SRS reduced overall brain recurrence rates from 76% to 47%. As discussed above, this improvement in intracranial cancer control did not translate into a survival benefit or a reduction in neurologic death. This confirmed the observation from a large multi-center retrospective study (46) that adding WBRT to SRS did not improve survival. Of note, new brain metastases can be easily managed with a second course of SRS, which may obviate any benefit from an initial improvement in intracranial control.
SRS instead of WBRT
SRS has emerged as an alternative to WBRT, because the later: (I) yields disappointing rates of local control; (II) may have deleterious effects (acute decline in QOL and memory, and possible late dementia); and (III) delays systemic therapy. Using the GammaKnife platform, numerous lesions can be safely treated in a single session. As described above, Aoyama’s randomized study (36) of patients with 1-4 brain metastases showed that the addition of WBRT to SRS reduced overall brain recurrence rates from 76% to 47%, but afforded no improvement in rates of survival or neurologic death. The same trial (47) showed the improved control of brain metastases seen with WBRT + SRS was associated with better neurocognitive function one and two years following treatment. However, in 3-year survivors, neurocognitive function may have been adversely affected by WBRT. While Aoyama concluded that WBT could be safely omitted in patients with 1-4 metastatic deposits, an alternate conclusion (48) is that WBRT was beneficial, as it decreased tumor recurrence without adversely effecting neurocognitive function (at least though year two). In an ECOG trial (49) of 36 patients with 1-3 histologically radioresistant metastases (renal cell, melanoma, sarcoma), intracranial failure was 48% 6 months after SRS alone. The authors concluded that “routine avoidance of WBRT should be approached judiciously”. In conclusion, there is no clear consensus when SRS alone is appropriate in brain metastases. In patients with 1-4 metastases, the addition of WBRT to SRS improves both local and distant intracranial control, but does not appear to affect survival. Although the improved intracranial control may delay neurocognitive decline, in long-term survivors WBRT may harm neurocognition. In addition, learning and memory appears to be adversely affected four months after WBRT.
More than four metastases
For decades WBRT has been standard therapy for patient with numerous brain metastases. In patients with more than four brain metastases, no randomized data is available to compare the efficacy of WBRT with radiosurgery. For the reasons outlined above, SRS is an attractive alternative to WBRT in good prognosis patients with more than four metastases. In a study of 205 patients with four or more brain metastases treated with SRS at the University of Pittsburgh (33), multivariate analysis showed lower total treatment volume, but not fewer metastases, was associated with improved local control. A large prospective multi-center study (50) found no difference in survival or in toxicity in SRS patients with 5-10 metastases, versus fewer. Finally, a large retrospective multicenter analysis (46) which included patients with numerous metastases showed that adding WBRT to SRS had no impact on overall survival. Thus SRS alone appears to be a reasonable alternative to WBRT in patients with KPS ≥70 with more than four brain metastases. A randomized study to compare these approaches is needed.
Post-resection SRS
Intracranial recurrence rates following surgical resection of brain metastases are substantial. Two prospective randomized trials (28,51) demonstrated that the addition of WBRT following resection improved intracranial disease control, without impacting overall survival. Patchell showed that adding WBRT following resection reduced 1-year intracranial recurrence rates from 70% to 18%. The greatest benefit of WBRT was in preventing local relapse: cavity recurrence was reduced from 46% to 10%, while elsewhere brain recurrences were reduced from 37% to 14%. Since WBRT may detrimentally affect learning/memory function and QOL, and may have serious late sequelae, local SRS to the resection cavity has been advocated. Several series (52-56) have shown that either single fraction or multiple fraction SRS yields local control rates similar to those seen with WBRT. With this approach, subclinical disease elsewhere in the brain is not addressed, thus distant brain recurrences are more frequent. Regular surveillance MRI scanning effectively diagnoses new brain metastases when they are small in size and readily addressed with repeat radiosurgical procedures.
SRS salvage following WBRT
The early reports (57) of SRS in treating brain metastases were in recurrences following WBRT. The dose escalation study (58) which established safe SRS dosing (RTOG 90-05) treated mostly recurrent brain metastases. One-year local control rates following SRS salvage are around 70-90% (59); toxicity is acceptable. SRS is now standard therapy for intracranial recurrences following WBRT in patients with appropriate life expectancy.
Follow-up imaging
Patients with brain metastases are at risk for subsequent intracranial failure. In Aoyama’s study (36), by one year, new brain metastases occurred in 64% of patients following SRS alone, and in 42% of patients treated with WBRT and SRS. Since salvage SRS is safe and effective, patients should be followed closely, with brain MRI scans obtained every three months after treatment.
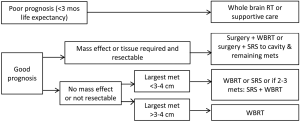
Treatment algorithms by subgroup
Algorithms for the treatment of brain metastases are indicated in Figures 1 and 2 below.
Poor prognosis
In patient expected to live less than 3 months, reasonable management options include best supportive care, or WBRT.
Treatment of solitary brain metastasis (see Figure 1)
Class 1 (prospective randomized) evidence shows that surgery or SRS improves overall survival in favorable prognosis patients with solitary brain metastases. If surgical resection is feasible, this is preferred in patients with mass effect, or when a tissue diagnosis is required, or in large tumors. Surgery or SRS is appropriate for patients with solitary metastases less than 3-4 cm in diameter. In all patients undergoing surgical resection, post-operative therapy is required: either WBRT or more localized fractionated radiotherapy or SRS. In patients undergoing SRS for solitary metastases, adding WBRT is optional.
Treatment of multiple brain metastases (see Figure 2)
In good prognosis patients, surgery may be beneficial to relieve mass effect, or if a tissue diagnosis is required. In other patients, if the largest metastasis is less than 3-4 cm, then SRS or WBRT are options. RTOG 9508 supports adding SRS to WBRT in patients with 2-3 metastases. In patients with 2-4 metastases, whether SRS alone is sufficient remains subject to debate: adding WBRT improves intracranial control, which may translate into better neurocognitive function, although WBRT appears to detrimentally learning and memory function as early as 4 months after treatment. Prospective randomized data is lacking in patients with more than four metastases, however retrospective studies suggest SRS alone is an appropriate alternative to WBRT in good prognosis patients. Thus a personalize approach taking into account the impact of WBRT on the patient’s individual situation is advisable.
Conclusions
Focal therapies for brain metastases have emerged as an important addition to WBRT in managing brain metastases. In patients with KPS ≥70 and with a single brain metastasis, either SRS or surgical resection should be offered, as this intervention improves overall survival. For patients with KPS ≥70 and 2-3 metastases, an SRS boost should be considered following WBRT, as this improves subsequent performance status, and in RPA class 1 patients, improves survival. Post-operative radiotherapy (WBRT or resection cavity SRS or RT) is mandatory following surgical resection. SRS alone may be a suitable alternative to WBRT in patients with a good prognosis and with tumors less than 3-4 cm in diameter. Adding WBRT to SRS improves intracranial control rates, but does not impact overall survival, as new metastases can be readily salvaged with SRS. Since WBRT acutely affects QOL, delays chemotherapy, and reduces learning and memory function, many centers advocate SRS alone as upfront treatment for brain metastases. Such patients should subsequently undergoing regular surveillance MRI scanning.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Sandra Vermuelen, Kevin T. Murphy, Huan Giap) for the series “SBRT/SRS in Radiation Research” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2014.08.03). The series “SBRT/SRS in Radiation Research” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29. [PubMed]
- Posner J. Brain metastases. In: Brain metastasis. Netherlands: Springer, 1980:2-29.
- Pickren JW, Lopez G, Tsukada Y, et al. Brain metastases: an autopsy study. Cancer Treat 1983;2:295-313.
- Walker AE, Robins M, Weinfeld FD. Epidemiology of brain tumors: the national survey of intracranial neoplasms. Neurology 1985;35:219-26. [PubMed]
- Schouten LJ, Rutten J, Huveneers HA, et al. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer 2002;94:2698-705. [PubMed]
- Barnholtz-Sloan JS, Sloan AE, Davis FG, et al. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol 2004;22:2865-72. [PubMed]
- Gavrilovic IT, Posner JB. Brain metastases: epidemiology and pathophysiology. J Neurooncol 2005;75:5-14. [PubMed]
- Delattre JY, Krol G, Thaler HT, et al. Distribution of brain metastases. Arch Neurol 1988;45:741-4. [PubMed]
- Lohr F, Pirzkall A, Hof H, et al. Adjuvant treatment of brain metastases. Semin Surg Oncol 2001;20:50-6. [PubMed]
- Posner JB. Clinical manifestations of brain metastases. In: Weiss L, Gilbert HA, Posner JB. eds. Brain Metastases. Boston: GK Hall, 1980:189-207.
- Price AC, Runge VM, Babigian GV. Brain: Neoplastic disease. In: Runge VM. eds. Clinical Magnetic Resonance Imaging. Philadelphia: JB Lippincott, 1990:113-75.
- Williams AL. Tumors p.16. In: Williams AL, Haughton VM. eds. Cranial Computed Tomography: A Comprehensive Text. St Louis: Mosby, 1985:17-29.
- Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 1990;322:494-500. [PubMed]
- Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys 1997;37:745-51. [PubMed]
- Ehrenkranz JR, Posner JB. Adrenocorticosteroid hormones. In: Weiss L, Gilbert HA, Posner JB. eds. Brain Metastases. Boston: Hall, 1980:340-63.
- Fishman RA. Brain edema. N Engl J Med 1975;293:706-11. [PubMed]
- Langley RE, Stephens RJ, Nankivell M, et al. Interim data from the Medical Research Council QUARTZ Trial: does whole brain radiotherapy affect the survival and quality of life of patients with brain metastases from non-small cell lung cancer? Clin Oncol (R Coll Radiol) 2013;25:e23-30. [PubMed]
- Horton J, Baxter DH, Olson KB. The management of metastases to the brain by irradiation and corticosteroids. Am J Roentgenol Radium Ther Nucl Med 1971;111:334-6. [PubMed]
- Glantz MJ, Cole BF, Forsyth PA, et al. Practice parameter: anticonvulsant prophylaxis in patients with newly diagnosed brain tumors. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2000;54:1886-93. [PubMed]
- Noordijk EM, Vecht CJ, Haaxma-Reiche H, et al. The choice of treatment of single brain metastasis should be based on extracranial tumor activity and age. Int J Radiat Oncol Biol Phys 1994;29:711-7. [PubMed]
- Kurtz JM, Gelber R, Brady LW, et al. The palliation of brain metastases in a favorable patient population: a randomized clinical trial by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys 1981;7:891-5. [PubMed]
- Harwood AR, Simson WJ. Radiation therapy of cerebral metastases: a randomized prospective clinical trial. Int J Radiat Oncol Biol Phys 1977;2:1091-4. [PubMed]
- Murray KJ, Scott C, Greenberg HM, et al. A randomized phase III study of accelerated hyperfractionation versus standard in patients with unresected brain metastases: a report of the Radiation Therapy Oncology Group (RTOG) 9104. Int J Radiat Oncol Biol Phys 1997;39:571-4. [PubMed]
- Suh JH, Stea B, Nabid A, et al. Phase III study of efaproxiral as an adjunct to whole-brain radiation therapy for brain metastases. J Clin Oncol 2006;24:106-14. [PubMed]
- Borgelt B, Gelber R, Kramer S, et al. The palliation of brain metastases: final results of the first two studies by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys 1980;6:1-9. [PubMed]
- Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 2004;363:1665-72. [PubMed]
- Langley RE, Stephens RJ, Nankivell M, et al. Interim data from the Medical Research Council QUARTZ Trial: does whole brain radiotherapy affect the survival and quality of life of patients with brain metastases from non-small cell lung cancer? Clin Oncol (R Coll Radiol) 2013;25:e23-30. [PubMed]
- Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA 1998;280:1485-9. [PubMed]
- Slotman BJ, Mauer ME, Bottomley A, et al. Prophylactic cranial irradiation in extensive disease small-cell lung cancer: short-term health-related quality of life and patient reported symptoms: results of an international Phase III randomized controlled trial by the EORTC Radiation Oncology and Lung Cancer Groups. J Clin Oncol 2009;27:78-84. [PubMed]
- Welzel G, Fleckenstein K, Schaefer J, et al. Memory function before and after whole brain radiotherapy in patients with and without brain metastases. Int J Radiat Oncol Biol Phys 2008;72:1311-8. [PubMed]
- Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol 2009;10:1037-44. [PubMed]
- Doyle DM, Einhorn LH. Delayed effects of whole brain radiotherapy in germ cell tumor patients with central nervous system metastases. Int J Radiat Oncol Biol Phys 2008;70:1361-4. [PubMed]
- Bhatnagar AK, Flickinger JC, Kondziolka D, et al. Stereotactic radiosurgery for four or more intracranial metastases. Int J Radiat Oncol Biol Phys 2006;64:898-903. [PubMed]
- Varlotto JM, Flickinger JC, Niranjan A, et al. The impact of whole-brain radiation therapy on the long-term control and morbidity of patients surviving more than one year after gamma knife radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys 2005;62:1125-32. [PubMed]
- Serizawa T, Ono J, Iichi T, et al. Gamma knife radiosurgery for metastatic brain tumors from lung cancer: a comparison between small cell and non-small cell carcinoma. J Neurosurg 2002;97:484-8. [PubMed]
- Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA 2006;295:2483-91. [PubMed]
- Varlotto JM, Flickinger JC, Niranjan A, et al. The impact of whole-brain radiation therapy on the long-term control and morbidity of patients surviving more than one year after gamma knife radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys 2005;62:1125-32. [PubMed]
- Muacevic A, Kreth FW, Horstmann GA, et al. Surgery and radiotherapy compared with gamma knife radiosurgery in the treatment of solitary cerebral metastases of small diameter. J Neurosurg 1999;91:35-43. [PubMed]
- Schöggl A, Kitz K, Reddy M, et al. Defining the role of stereotactic radiosurgery versus microsurgery in the treatment of single brain metastases. Acta Neurochir 2000;142:621-6. [PubMed]
- O’Neill BP, Iturria NJ, Link MJ, et al. A comparison of surgical resection and stereotactic radiosurgery in the treatment of solitary brain metastases. Int J Radiat Oncol Biol Phys 2003;55:1169-76. [PubMed]
- Muacevic A, Wowra B, Siefert A, et al. Microsurgery plus whole brain irradiation versus Gamma Knife surgery alone for treatment of single metastases to the brain: a randomized controlled multicentre phase III trial. J Neurooncol 2008;87:299-307. [PubMed]
- Sanghavi SN, Miranpuri SS, Chappell R, et al. Radiosurgery for patients with brain metastases: a multi-institutional analysis, stratified by the RTOG recursive partitioning analysis method. Int J Radiat Oncol Biol Phys 2001;51:426-34. [PubMed]
- Stafinski T, Jhangri GS, Yan E, et al. Effectiveness of stereotactic radiosurgery alone or in combination with whole brain radiotherapy compared to conventional surgery and/or whole brain radiotherapy for the treatment of one or more brain metastases: a systematic review and meta-analysis. Cancer Treatment Reviews 2006;32:203-13. [PubMed]
- Rades D, Kueter JD, Hornung D, et al. Comparison of stereotactic radiosurgery (SRS) alone and (WBRT) plus a stereotactic boost (WBRT + SRS) for one to three brain metastases. Strahlenther Onkol 2008;184:655-62. [PubMed]
- Sneed PK, Lamborn KR, Forstner JM, et al. Radiosurgery for brain metastases: is whole brain radiotherapy necessary? Int J Radiat Oncol Biol Phys 1999;43:549-58. [PubMed]
- Sneed PK, Suh JH, Goetsch SJ, et al. A multi-institutional review of radiosurgery alone vs. radiosurgery with whole brain radiotherapy as the initial management of brain metastases. Int J Radiat Oncol Biol Phys 2002;53:519-26. [PubMed]
- Aoyama H, Tago M, Kato N, et al. Neurocognitive function of patients with brain metastasis who received either whole brain radiotherapy plus stereotactic radiosurgery or radiosurgery alone. Int J Radiat Oncol Biol Phys 2007;68:1388-95. [PubMed]
- Patchell RA, Regine WF, Loeffler JS, et al. Radiosurgery plus whole-brain radiation therapy for brain metastases. JAMA 2006;296:2089-90; author reply 2090-1. [PubMed]
- Manon R, O’Neill A, Knisely J, et al. Phase II trial of radiosurgery for one to three newly diagnosed brain metastases from renal cell carcinoma, melanoma, and sarcoma: an Eastern Cooperative Oncology Group study (E 6397). J Clin Oncol 2005;23:8870-6. [PubMed]
- Yamamoto M, Serizawa T, Shuto T, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol 2014;15:387-95. [PubMed]
- Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol 2011;29:134-41. [PubMed]
- Soltys SG, Adler JR, Lipani JD, et al. Stereotactic radiosurgery of the postoperative resection cavity for brain metastases. Int J Radiat Oncol Biol Phys 2008;70:187-93. [PubMed]
- Jagannathan J, Yen CP, Ray DK, et al. Gamma Knife radiosurgery to the surgical cavity following resection of brain metastases. J Neurosurg 2009;111:431-8. [PubMed]
- Hwang SW, Abozed MM, Hale A, et al. Adjuvant Gamma Knife radiosurgery following surgical resection of brain metastases: a 9-year retrospective cohort study. J Neurooncol 2010;98:77-82. [PubMed]
- Kelly PJ, Lin YB, Yu AY, et al. Stereotactic irradiation of the postoperative resection cavity for brain metastasis: a frameless linear accelerator-based case series and review of the technique. Int J Radiat Oncol Biol Phys 2012;82:95-101. [PubMed]
- Jensen CA, Chan MD, McCoy TP, et al. Cavity-directed radiosurgery as adjuvant therapy after resection of a brain metastasis. J Neurosurg 2011;114:1585-91. [PubMed]
- Loeffler JS, Kooy HM, Wen PY, et al. The treatment of recurrent brain metastases with stereotactic radiosurgery. J Clin Oncol 1990;8:576-82. [PubMed]
- Shaw E, Scott C, Souhami L, et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys 2000;47:291-8. [PubMed]
- Noël G, Proudhom MA, Valery CA, et al. Radiosurgery for re-irradiation of brain metastasis: results in 54 patients. Radiother Oncol 2001;60:61-7. [PubMed]