A retrospective analysis of the correlation between AXL expression and clinical outcomes of patients with urothelial bladder carcinoma
Introduction
Urothelial bladder carcinoma (BC) is a common malignancy in genitourinary tract worldwide, with an estimated 81,190 new cases and 17,240 deaths in USA, 2018 (1). Approximately 70–75% of newly diagnosed patients are non-muscle invasive BC (NMIBC) and 25–30% are invasive (MIBC) (2). Although NMIBC patients have a better prognosis than MIBC, 15–70% of NMIBC will recur, and a significant proportion of high-risk NMIBC patients will develop into MIBC within 5 years (3,4). The current prognostic models for NMIBC rely on pathologic features, which are obtained after invasive surgical examination and do not reach sufficient accuracy to identify the patients most likely to benefit from early radical cystectomy (RC) (5). Therefore, there is an urgent need to identify effective markers for predicting the prognosis of BC patients.
AXL, a member of the TAM (Tyro3, Axl, MerTK) family of receptor tyrosine kinase (RTKs), is involved in the regulation of cell survival, adhesion, and migration in many cancers, such as prostate cancer (6). In BC, it was reported that AXL depletion significantly inhibited the migration of tumor cells (7,8). Multiple and selective targeted tyrosine kinase inhibitors have a blocking effect on the progression of BC cells (9). Most AXL signaling occurs in a Gas6-mediated ligand-dependent manner (10). When bound to its ligand Gas6, AXL will induce the multiple downstream pathways, which has been found to play an important role in cancer development and progression (11,12).
Immunohistochemistry (IHC) analysis of primary tumors revealed that AXL expression is associated with metastasis and/or poor survival in patients with different cancers, such as breast cancer, hepatocellular and renal cell carcinoma (13-15). Few studies about prognostic value of AXL in BC have been published and the role of AXL in BC is still unclear. IHC in Yeh et al.’s study showed that AXL is non-significantly related to the clinical features and survivals of BC patients, but Hattori et al.’s study showed that AXL is associated with cancer-specific survival (CSS) of patients (8,16). The objective of our study is to investigate the prognostic value of AXL in predicting the clinical outcome of BC patients, which may help risk-based individuals adopt better treatment strategies.
Methods
TCGA database
The Cancer Genome Atlas (TCGA; https://cancergenome.nih.gov), an online bioinformatics database, provides extensive biological information for different carcinomas, including BC (17,18). A total of 407 BC patients with AXL mRNA expressional data (mRNASeq-count.txt) and sufficient clinical information to be used for analysis were obtained from TCGA database in our study.
Patients and specimens
A total of 203 patients with clinical and pathological diagnosis with BC in the 2nd hospital of Tianjin medical university (Tianjin, China) from June 2011 to December 2014 were included in our research. Tumor tissue of patients was obtained after first surgical treatment. Clinical data including the age, gender, smoking state, tumor grade, size, number, pathological T-stage (pT), lymph node metastasis (pN) and recurrence rate of patients were retrospectively recorded. Tumors were classified according to 2009 UICC TNM staging and 2004 WHO/ISUP classification (19,20). Overall survival (OS) of patients was followed-up. The whole study was approved by Human Ethics Committee of Tianjin medical university (Tianjin, China) (No.KY2016K010) and written informed consent was obtained from all participants. All patient’s personal data has been protected.
IHC
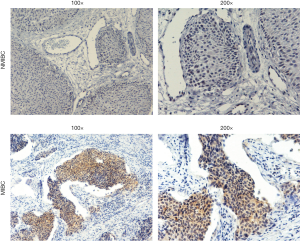
Paraffin-embedded tissues collected after surgical treatment were sliced into 4 µm sections and heated for 30 min at 65 °C. Then, tissue sections were performed with EDTA (pH =8.0) and 3% hydrogen peroxide in methanol. Slides were incubated with AXL Polyclonal Antibody (HPA037422, Sigma-Aldrich, USA) at 4 °C overnight. The second antibody (Solarbio, USA) was then incubated at room temperature for 30 min. For each IHC sample, five random fields were selected at low magnification (100×) and evaluated at high magnification (200×). The process was finished by two workers independently. For the results, H-sore method was applied to evaluate the expression of AXL protein (21). The staining intensity was divided as follows: no staining as 0; weak staining as 1; moderate staining as 2; strong staining as 3. The percentages of positive cells were categorized as follows: no staining as 0; 1–25% of stained cells as 1; 25–50% as 2; 51–85% as 3; 85–100% as 4. The score was calculated as follows: staining index = staining intensity × percentages of positive cells. The cutoff value of AXL expression was determined by distribution of scores.
Statistical analysis
All data were analyzed by SPSS.20.0 statistics software. Quantitative date was evaluated by mean ± SD. According to whether the variance was homogeneity, parameter test and non-parametric test were used respectively. Relationship between the expression of AXL and the clinicopathological features was evaluated using χ2 tests. The prognostic value of AXL was estimated by Kaplan-Meier method and Cox-regression analysis. For the results, P<0.05 for the difference was considered as significant.
Results
Relationship between expression of AXL gene mRNA and clinical outcomes of patients with BC from TCGA database
A total of 407 of BC samples from TCGA database were used to investigate the relationship between AXL expression and clinical-pathological characters of BC patients. AXL was associated with the patient’s age, pT, pN, and grade of tumors. Patients over 75-year-old were more likely to have high AXL expression (P<0.001). Increased AXL was associated with a worse pathological tumor grade, pT, and pN (P<0.05) (Table 1). Therefore, high expression of AXL was related to the unfavorable pathological features of BC patients.
Table 1
| Clinical-pathological features | AXL mRNA expression | OR | 95% CI | P value | ||
|---|---|---|---|---|---|---|
| Low | High | Total | ||||
| Age | ||||||
| <75 | 190 | 106 | 296 | 13.51 | 7.23–25.25 | <0.001* |
| ≥75 | 13 | 98 | 111 | |||
| Sex | ||||||
| Male | 156 | 144 | 300 | 1.38 | 0.89–2.16 | 0.152 |
| Female | 47 | 60 | 107 | |||
| Race | ||||||
| White | 148 | 175 | 323 | N | N | 0.008* |
| Asian | 31 | 13 | 44 | |||
| Black | 10 | 13 | 23 | |||
| pT | ||||||
| T2 | 70 | 49 | 119 | N | N | 0.01* |
| T3 | 81 | 112 | 193 | |||
| T4 | 25 | 33 | 58 | |||
| pN | ||||||
| No | 127 | 110 | 237 | 1.86 | 1.20–2.89 | 0.005* |
| Yes | 49 | 79 | 128 | |||
| Grade | ||||||
| Low | 16 | 5 | 21 | 3.43 | 1.23–9.54 | 0.013* |
| High | 185 | 198 | 383 | |||
| Recurrence | ||||||
| Yes | 90 | 83 | 173 | 1.22 | 0.78–1.90 | 0.391 |
| No | 66 | 74 | 140 | |||
*, P<0.05. BC, bladder carcinoma; TCGA, The Cancer Genome Atlas; OR, odds ratio; N, no odds ratio analysis was performed in the multi-group analysis; pT, pathological T-stages; pN, lymph node metastasis.
For prognostic value, univariate and multivariate analyses were performed to evaluate the relationship between these features and the OS of patients. In univariate analysis, high expression of AXL was associated with short OS (P=0.048); however, there was no significant difference in multivariate analysis (P=0.218). In addition, tumor pT and pN were significantly associated with OS (P<0.01) (Table 2). In conclusion, from TCGA database, we found that AXL was associated with poor clinical outcomes in BC patients.
Table 2
| Clinical features | Median | Univariate | Multivariate | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |||
| Age | ||||||
| <75 | 35.3 | 1.372 (0.999–1.885) | 0.051 | 1.253 (0.881–1.783) | 0.210 | |
| ≥75 | 26.9 | |||||
| Sex | ||||||
| Male | 34.9 | 1.123 (0.811–1.555) | 0.485 | 1.210 (0.836–1.752) | 0.313 | |
| Female | 30.9 | |||||
| Race | ||||||
| White | 32 | 0.984 (0.742–1.305) | 0.912 | 1.185 (0.873–1.608) | 0.277 | |
| Asian | 54.8 | |||||
| Black | 22.8 | |||||
| pT | ||||||
| T2 | 86.7 | 1.731 (1.376–2.177) | <0.001* | 1.483 (1.134–1.939) | 0.004* | |
| T3 | 27 | |||||
| T4 | 17.1 | |||||
| pN | ||||||
| No | 64.7 | 2.233 (1.629–3.060) | <0.001* | 1.819 (1.281–2.582) | 0.001* | |
| Yes | 19.4 | |||||
| Grade | ||||||
| Low | 29.7 | 2.965 (0.734–11.985) | 0.109 | 2.645 (0.358–19.546) | 0.341 | |
| High | 32.9 | |||||
| AXL | ||||||
| Low | 44.2 | 1.349 (1.003–1.816) | 0.048* | 1.273 (0.882–1.735) | 0.218 | |
| High | 28.2 | |||||
*, P<0.05. OS, overall survival; BC, bladder carcinoma; TCGA, The Cancer Genome Atlas; HR, hazard ratio; pT, pathological T-stages; pN, lymph node metastasis.
AXL was associated with clinical-pathological features of BC patients
A total of 203 patients diagnosed with BC were included in our study. The details of these patients are summarized in Table 3. IHC was performed to assess the expression of AXL in tumor tissues. The typical staining of AXL is shown in Figure 1. Consistent with TCGA, our results showed that abundant AXL was significantly related to worse pathological tumor grade, pT and pN of patients (P<0.05). However, there was also a correlation between AXL and patients’ gender (P=0.018) and tumor size (P=0.021) (Table 3).
Table 3
| Characters | Numbers | Percentage | AXL expression | |||
|---|---|---|---|---|---|---|
| Low | High | OR (95% CI) | P value | |||
| Ages | ||||||
| <70 | 118 | 58.1 | 66 | 52 | 1.08 (0.61–1.89) | 0.798 |
| ≥70 | 85 | 41.9 | 46 | 39 | ||
| Gender | ||||||
| Male | 170 | 83.7 | 100 | 70 | 2.50 (1.16–5.41) | 0.018* |
| Female | 33 | 16.3 | 12 | 21 | ||
| Smoking | ||||||
| No | 114 | 56.2 | 66 | 48 | 1.29 (0.74–2.25) | 0.377 |
| Yes | 89 | 43.8 | 46 | 43 | ||
| Grade | ||||||
| Low | 79 | 38.9 | 62 | 17 | 5.40 (2.83–10.29) | <0.001* |
| High | 124 | 61.1 | 50 | 74 | ||
| pT | ||||||
| Ta, T1 | 117 | 57.6 | 79 | 38 | 3.34 (1.87–5.98) | <0.001* |
| T2–T4 | 86 | 42.4 | 33 | 53 | ||
| pN | ||||||
| No | 174 | 85.7 | 102 | 72 | 2.69 (1.18–6.13) | 0.016* |
| Yes | 29 | 14.3 | 10 | 19 | ||
| Ki67 | ||||||
| Low | 102 | 50.2 | 58 | 44 | 1.15 (0.66–2.00) | 0.626 |
| High | 101 | 49.8 | 54 | 47 | ||
| Tumor sizes | ||||||
| <2 cm | 114 | 56.2 | 71 | 43 | 1.93 (1.10–3.40) | 0.021* |
| ≥2 cm | 89 | 43.8 | 41 | 48 | ||
| Tumor numbers | ||||||
| Single | 85 | 41.9 | 45 | 40 | 0.86 (0.49–1.50) | 0.587 |
| Multiple | 118 | 58.1 | 67 | 51 | ||
| Recurrence | ||||||
| No | 117 | 57.6 | 62 | 55 | 0.81 (0.46–1.42) | 0.466 |
| Yes | 86 | 42.4 | 50 | 36 | ||
*, P<0.05. BC, bladder carcinoma; OR, odds ratio; pT, pathological T-stages; pN, lymph node metastasis.
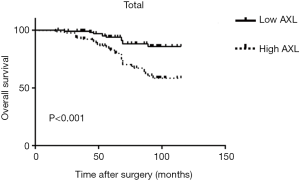
High expression of AXL was related to a worse OS of BC patients
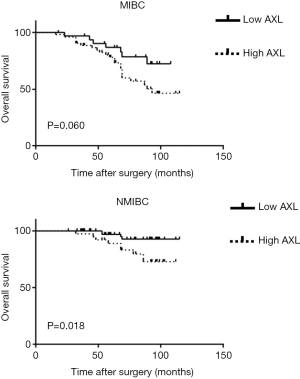
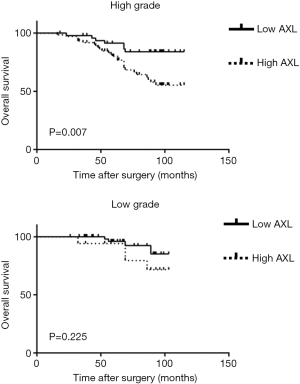
Kaplan-Meier analysis was performed to investigate prognostic value of AXL in OS of patients. High AXL was significantly associated with a short OS (P<0.001) (Figure 2). Then, we divided the samples into different groups according to tumor grade and pT. AXL was related to the OS in patients with NMIBC (P=0.018), but not MIBC (P=0.060) (Figure 3). There was a relationship between AXL and OS in patients with high tumor grade (P=0.007), but not low tumor grade (P=0.225) (Figure 4).
In univariate analysis, pathological tumor grade, pT, PN, Ki67 and AXL were significantly associated with a worse OS (P<0.05). In multivariate analysis, AXL, as well as pathological pT, pN, and Ki67, was an independent prognostic factor in predicting the survival of BC patients (P<0.05) (Table 4).
Table 4
| Characters | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Ages | 1.850 | 1.007–3.398 | 0.047* | 1.730 | 0.911–3.287 | 0.094 | |
| Genders | 1.826 | 0.917–3.636 | 0.087 | – | – | – | |
| Smoking | 0.865 | 0.463–1.616 | 0.650 | – | – | – | |
| Grade | 2.399 | 1.109–5.190 | 0.026* | 1.082 | 0.480–2.439 | 0.849 | |
| pT | 3.345 | 1.739–6.437 | <0.001* | 2.131 | 1.057–4.297 | 0.034* | |
| pN | 6.567 | 3.555–12.131 | <0.001* | 4.369 | 2.288–8.342 | <0.001* | |
| Ki67 | 1.979 | 1.053–3.722 | 0.034* | 2.366 | 1.222–4.581 | 0.011* | |
| Tumor sizes | 0.718 | 0.388–1.328 | 0.291 | – | – | – | |
| Tumor numbers | 1.301 | 0.697–2.430 | 0.408 | – | – | – | |
| AXL | 3.328 | 1.673–6.624 | 0.001* | 2.193 | 1.058–4.545 | 0.035* | |
*, P<0.05. OS, overall survival; BC, bladder carcinoma; HR, hazard ratio; pT, pathological T-stages; pN, lymph node metastasis.
Discussion
With the high rates of recurrence, short progress and death time, monitoring BC patients’ survival is extremely important and brings a giant economic burden worldwide (22). Despite the combination of RC and chemotherapy, only 30–40% of patients with MIBC survive 5 years or longer (23). Cystoscopy is the standard diagnostic tool of BC, but it is expensive, time consuming, invasive and leads to infection in more than 16% of patients (24). Many biomarkers have been found to play a role in BC and could predict the survival of patients (25). However, the strategy based on these markers was unsatisfied. Therefore, novel biomarkers need to be researched.
RTK plays a key role in cell signaling and is involved in tumor progression. In 1991, AXL was first identified as an RTK in patients with chronic myeloid leukemia (26). The TAM family of RTKs comprises three transmembrane receptors: TYRO-3, AXL, and MER. There are two main ligands for TAM receptors, growth arrest-specific gene 6 (Gas6) and protein S (PROS) (27,28). In the past decades, the TAM family has become an important factor in controlling tissue homeostasis and innate immune responses. Dysregulation of TAM signaling is associated with chronic inflammation, autoimmune diseases and cancers (29).
Overexpression of AXL has been found in multiple carcinomas and is often associated with poor prognosis (10,30). In our study, we first used TCGA data to analyze the relationship between AXL expression and clinical characters of BC patients. The results showed that AXL expression was associated with the poor clinical-pathological characters, such as T-stage, pN, and tumor grade, and a worse OS of patients. Then, 203 samples from our hospital were used to research the prognostic value of AXL. Consistent with the result of TCGA, we found that abundant AXL was related to the poor clinical outcomes. AXL was an independent prognostic factor in predicting the survival of BC patients. However, in our study, the relationship between AXL expression and the survival of MIBC patients was non-significant (P=0.06). The difference of results between our study and TCGA might be due to the sample size limit in our study [86], but 407 MIBC samples were included in TCGA database. Therefore, a large sample number and multi-center study is needed in the future.
In the previous Hattori et al.’s study, the results showed that AXL were independently associated with lower CSS. In a subgroup analysis of patients with NMIBC, no significant difference in CSS was observed between patients with weak expression and strong expression of AXL (31). However, in the current study, our results exhibited that the expression of AXL was significantly related to OS in patients with NMIBC. The discrepancy may be due to differences in the type of specimens, the number of samples, or IHC assessment method. As a retrospective study, the sample size was small and all patients were obtained from only one center. In the future, a large sample multi-center research is necessary to confirm our conclusions. Other states, such as distant metastasis, intravesical Bacillus Calmette Guerin (BCG) treatment and chemotherapy, which may influence the survival of patients, were not analyzed by subgroup in our study. This may potentially cause discrepancy in results.
In conclusion, our results demonstrated that AXL expression was significantly associated with the prognosis of patients with BC. Patients with abundant AXL were more likely to have a poor clinical outcome. The result will contribute to better understand AXL as a biomarker for BC and provide a better strategy for clinical treatment.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.06.06). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The whole study was approved by Human Ethics Committee of Tianjin medical university (Tianjin, China) (No.KY2016K010) and written informed consent was obtained from all participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Agostinis P, Berg K, Cengel KA, et al. Photodynamic therapy of cancer: an update. CA Cancer J Clin 2011;61:250-81. [Crossref] [PubMed]
- Sanli O, Dobruch J, Knowles MA, et al. Bladder cancer. Nat Rev Dis Primers 2017;3:17022. [Crossref] [PubMed]
- Kurth KH, Denis L, Bouffioux C, et al. Factors affecting recurrence and progression in superficial bladder tumours. Eur J Cancer 1995;31A:1840-6. [Crossref] [PubMed]
- Babjuk M, Oosterlinck W, Sylvester R, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder. Eur Urol 2008;54:303-14. [Crossref] [PubMed]
- Jin X, Yun SJ, Jeong P, et al. Diagnosis of bladder cancer and prediction of survival by urinary metabolomics. Oncotarget 2014;5:1635-45. [Crossref] [PubMed]
- Rankin EB, Giaccia AJ. The Receptor Tyrosine Kinase AXL in Cancer Progression. Cancers 2016;8:103. [Crossref] [PubMed]
- Sayan AE, Stanford R, Vickery R, et al. Fra-1 controls motility of bladder cancer cells via transcriptional upregulation of the receptor tyrosine kinase AXL. Oncogene 2012;31:1493-503. [Crossref] [PubMed]
- Yeh CY, Shin SM, Yeh HH, et al. Transcriptional activation of the Axl and PDGFR-alpha by c-Met through a ras- and Src-independent mechanism in human bladder cancer. BMC Cancer 2011;11:139. [Crossref] [PubMed]
- Hänze J, Kessel F, Di Fazio P, et al. Effects of multi and selective targeted tyrosine kinase inhibitors on function and signaling of different bladder cancer cells. Biomed Pharmacother 2018;106:316-25. [Crossref] [PubMed]
- Brown M, Black JR, Sharma R, et al. Gene of the month: Axl. J Clin Pathol 2016;69:391-7. [Crossref] [PubMed]
- Lee HJ, Jeng YM, Chen YL, et al. Gas6/Axl pathway promotes tumor invasion through the transcriptional activation of Slug in hepatocellular carcinoma. Carcinogenesis 2014;35:769-75. [Crossref] [PubMed]
- Wang C, Jin H, Wang N, et al. Gas6/Axl Axis Contributes to Chemoresistance and Metastasis in Breast Cancer through Akt/GSK-3β/β-catenin Signaling. Theranostics 2016;6:1205-19. [Crossref] [PubMed]
- Gjerdrum C, Tiron C, Høiby T, et al. Axl is an essential epithelial-to-mesenchymal transition-induced regulator of breast cancer metastasis and patient survival. Proc Natl Acad Sci U S A 2010;107:1124-9. [Crossref] [PubMed]
- Liu J, Wang K, Yan Z, et al. Axl Expression Stratifies Patients with Poor Prognosis after Hepatectomy for Hepatocellular Carcinoma. Plos One 2016;11:e0154767. [Crossref] [PubMed]
- Zhou L, Liu XD, Sun M, et al. Targeting MET and AXL overcomes resistance to sunitinib therapy in renal cell carcinoma. Oncogene 2016;35:2687. [Crossref] [PubMed]
- Hattori S, Kikuchi E, Kosaka T, et al. Relationship Between Increased Expression of the Axl/Gas6 Signal Cascade and Prognosis of Patients with Upper Tract Urothelial Carcinoma. Ann Surg Oncol 2016;23:663-70. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 2014;507:315-22. [Crossref] [PubMed]
- Grossman RL, Heath AP, Ferretti V, et al. Toward a Shared Vision for Cancer Genomic Data. N Engl J Med 2016;375:1109-12. [Crossref] [PubMed]
- Webber C, Gospodarowicz M, Sobin LH, et al. Improving the TNM classification: findings from a 10-year continuous literature review. Int J Cancer 2014;135:371-8. [Crossref] [PubMed]
- Lokeshwar SD, Ruiz-Cordero R, Hupe MC, et al. Impact of 2004 ISUP/WHO classification on bladder cancer grading. World J Urol 2015;33:1929-36. [Crossref] [PubMed]
- Pirker R, Pereira JR, von Pawel J, et al. EGFR expression as a predictor of survival for first-line chemotherapy plus cetuximab in patients with advanced non-small-cell lung cancer: analysis of data from the phase 3 FLEX study. Lancet Oncol 2012;13:33-42. [Crossref] [PubMed]
- Kaufman DS, Shipley WU, Feldman AS. Bladder cancer. Lancet 2009;374:239-49. [Crossref] [PubMed]
- Alfred Witjes J, Lebret T, Compérat EM, et al. Updated 2016 EAU Guidelines on Muscle-invasive and Metastatic Bladder Cancer. Eur Urol 2017;71:462-75. [Crossref] [PubMed]
- Gkialas I, Papadopoulos G, Iordanidou L, et al. Evaluation of urine tumor-associated trypsin inhibitor, CYFRA 21-1, and urinary bladder cancer antigen for detection of high-grade bladder carcinoma. Urology 2008;72:1159-63. [Crossref] [PubMed]
- Rouprêt M, Babjuk M, Compérat E, et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2017 Update. Eur Urol 2018;73:111. [Crossref] [PubMed]
- O'Bryan JP, Frye RA, Cogswell PC, et al. axl, a transforming gene isolated from primary human myeloid leukemia cells, encodes a novel receptor tyrosine kinase. Mol Cell Biol 1991;11:5016-31. [Crossref] [PubMed]
- Mark MR, Chen J, Hammonds RG, et al. Characterization of Gas6, a member of the superfamily of G domain-containing proteins, as a ligand for Rse and Axl. J Biol Chem 1996;271:9785-9. [Crossref] [PubMed]
- Stitt TN, Conn G, Gore M, et al. The anticoagulation factor protein S and its relative, Gas6, are ligands for the Tyro 3/Axl family of receptor tyrosine kinases. Cell 1995;80:661-70. [Crossref] [PubMed]
- Lemke G. Biology of the TAM receptors. Cold Spring Harb Perspect Biol 2013;5:a009076. [Crossref] [PubMed]
- Zhang S, Xu XS, Yang JX, et al. The prognostic role of Gas6/Axl axis in solid malignancies: a meta-analysis and literature review. Onco Targets Ther 2018;11:509-19. [Crossref] [PubMed]
- Hattori S, Kikuchi E, Kosaka T, et al. Relationship Between Increased Expression of the Axl/Gas6 Signal Cascade and Prognosis of Patients with Upper Tract Urothelial Carcinoma. Ann Surg Oncol 2016;23:663-70. [Crossref] [PubMed]